Types of Reactions Worksheet: Balancing Answers Explained

Delving into the world of chemistry, one cannot overlook the fundamental concept of chemical reactions. These reactions are not just mere transformations of substances; they're the cornerstone of understanding how materials interact at the molecular level. Among the various aspects of chemical reactions, balancing equations stands out as an essential skill. A worksheet filled with balancing answers can help students master this skill. Here, we'll explore different types of chemical reactions, their balancing mechanisms, and provide insights into the answers that can clarify this topic for learners.
1. Introduction to Chemical Reactions

Chemical reactions occur when atoms of different substances rearrange to form new compounds. This process is guided by the law of conservation of mass, which states that the total mass of reactants must equal the total mass of products. Thus, every chemical equation must be balanced to reflect this law.

Chemical reactions are categorized based on:
- Combination or synthesis reactions
- Decomposition reactions
- Single displacement reactions
- Double displacement or precipitation reactions
- Combustion reactions
2. Balancing Chemical Equations

Balancing a chemical equation involves ensuring that the number of atoms of each element is the same on both the reactant and product sides. Here's the method:
- Write down the unbalanced equation
- Choose an element and balance its atoms
- Move to the next element, balancing without altering previously balanced elements
- Use coefficients to balance atoms
- Ensure hydrogen and oxygen are balanced last (for simplicity)
- Check your work by confirming all atoms are equal
Example of Balancing a Chemical Equation:
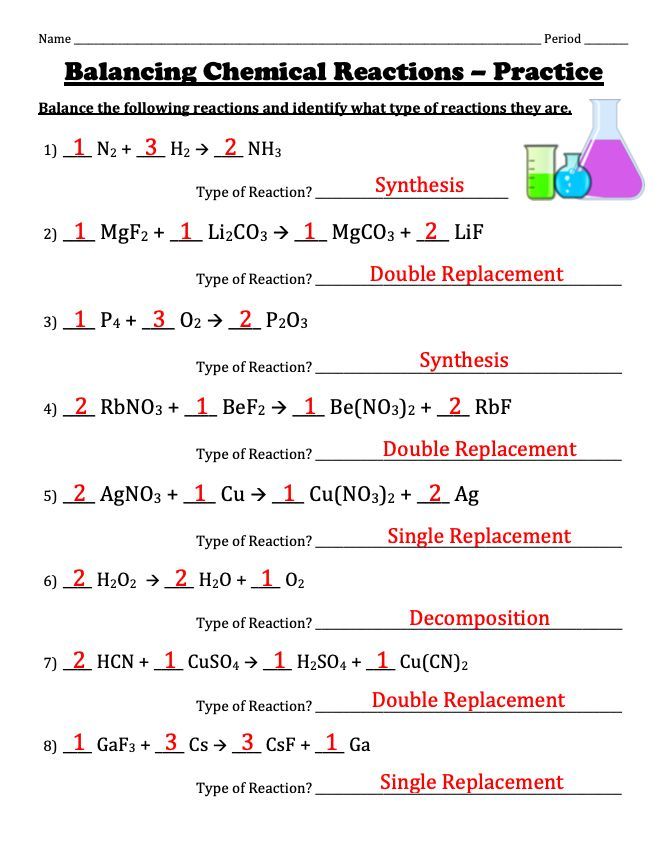
Let's consider the synthesis reaction:
N₂ + H₂ → NH₃
Here's how we balance it:
- Start with Nitrogen: N₂ + 3H₂ → 2NH₃
- Hydrogen must be balanced: Now, both sides have 6 hydrogen atoms
- The equation is balanced
3. Types of Reactions and Their Balancing

Combination or Synthesis Reactions

A synthesis reaction occurs when two or more substances combine to form a single product. Here’s an example:
| Reactants | Product | Balanced Equation |
|---|---|---|
| Na + Cl₂ | NaCl | 2Na + Cl₂ → 2NaCl |

To balance this reaction:
- Place a 2 in front of Na to balance the sodium atoms.
- Now both sides have 2 sodium atoms and 2 chlorine atoms, and the equation is balanced.
Decomposition Reactions

Decomposition reactions break down a compound into two or more simpler substances. Balancing these reactions often requires:
- Ensuring total mass is conserved.
- Balancing the product atoms first, then adjust reactant atoms.
Example:
CaCO₃ → CaO + CO₂
This equation is already balanced since there is 1 calcium, 1 carbon, and 3 oxygen atoms on both sides.
Single Displacement Reactions

Here, an element displaces another in a compound. Balancing steps:
- Identify the element being displaced.
- Balance based on atomic numbers first, then adjust the coefficients.
Example:
Zn + CuSO₄ → ZnSO₄ + Cu
This equation is balanced by nature.
Double Displacement or Precipitation Reactions

Two compounds exchange ions to form two new compounds. Steps to balance include:
- Identify the cations and anions.
- Balance the charges first, then the coefficients.
Example:
AgNO₃ + NaCl → AgCl + NaNO₃
This equation is already balanced as all ions are in a 1:1 ratio.
Combustion Reactions

Typically involve oxygen combining with a fuel to produce carbon dioxide and water. Balancing steps:
- Identify the hydrocarbon and its carbon and hydrogen atoms.
- Balance carbon atoms, then hydrogen, followed by oxygen.
Example:
CH₄ + O₂ → CO₂ + H₂O
To balance this:
- Balancing carbon: CH₄ + 2O₂ → CO₂ + 2H₂O
- Balancing hydrogen and oxygen: now the equation is balanced.
⚠️ Note: Always double-check your balanced equation, especially in combustion reactions where oxygen can have different coefficients.
In conclusion, mastering the art of balancing chemical equations is not only a cornerstone of chemistry but also a practical skill for scientific analysis. Understanding the different types of reactions provides the framework needed to balance equations efficiently. By following the steps provided for each type of reaction, students can systematically address this challenge and ensure conservation of mass in their chemical transformations.
Why is it important to balance chemical equations?

+
Balancing chemical equations is crucial because it demonstrates the law of conservation of mass, ensuring that the number of atoms for each element in the reactants equals the number of atoms in the products. This balance validates the integrity of the chemical reaction and its feasibility under scientific principles.
Can you have fractions when balancing chemical equations?

+
Yes, fractions can be used as coefficients in an intermediate step while balancing. However, chemists often multiply the entire equation by the lowest common multiple of the denominators to get rid of the fractions, ensuring whole number coefficients.
What’s the simplest approach to balance complex reactions?

+
Begin by balancing the most complex molecule or ion in the equation. Then, move to simpler molecules or ions, adjusting coefficients as necessary. This approach often helps in systematically tackling the balancing process, especially for reactions involving multiple elements or polyatomic ions.