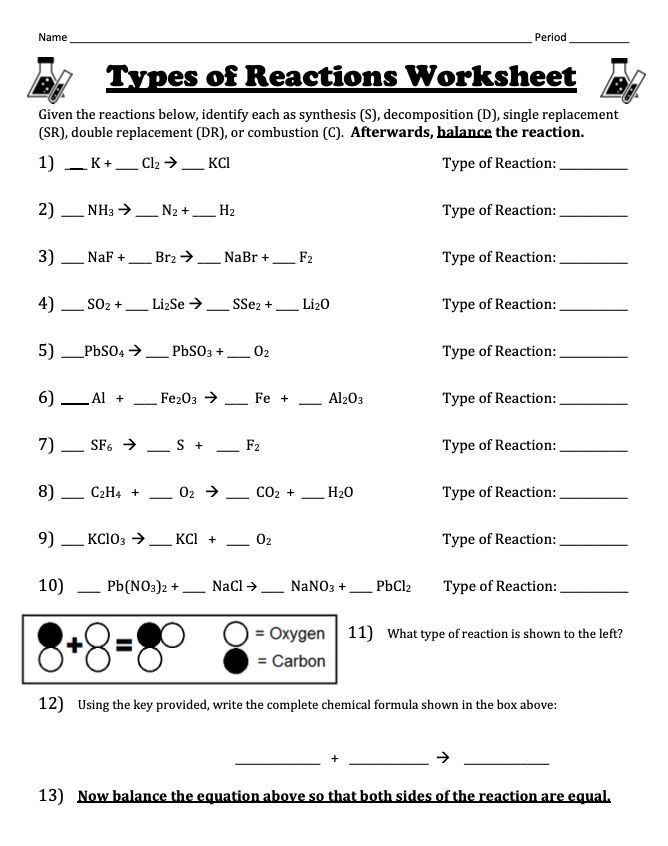
5 Types of Reactions: Worksheet & Balancing Guide

In the world of chemistry, understanding the types of chemical reactions is fundamental for students and enthusiasts alike. This exploration not only aids in the prediction of reaction outcomes but also in mastering the balance of chemical equations, a key aspect in chemical education. This article dives deep into five common types of chemical reactions, providing both a worksheet and a comprehensive guide on how to balance these reactions.
Understanding Chemical Reactions

Chemical reactions occur when substances interact, leading to a change in their chemical properties. These changes involve the breaking and forming of chemical bonds, transforming reactants into products. Here are the five key types of chemical reactions we'll discuss:
- Combination Reactions
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reactions
- Combustion Reactions
Combination Reactions

A combination reaction, often called a synthesis reaction, involves two or more substances combining to form a single compound. The general form is:
[ A + B \rightarrow AB ]
Here's a simple example:
[ 2H_2 + O_2 \rightarrow 2H_2O ]
⚗️ Note: For combination reactions, ensure that the charges of the ions are balanced when combining elements into compounds.
Decomposition Reactions


Decomposition is the reverse of combination, where a single compound breaks down into two or more elements or simpler compounds. The basic equation is:
[ AB \rightarrow A + B ]
An example includes:
[ 2H_2O \rightarrow 2H_2 + O_2 ]
🔬 Note: Decomposition often requires an input of energy such as heat or light to break the bonds.
Single Displacement Reactions


In this reaction, one element takes the place of another in a compound. It can be represented as:
[ A + BC \rightarrow AC + B ]
For instance:
[ Zn + CuSO_4 \rightarrow ZnSO_4 + Cu ]
⚠️ Note: Single displacement reactions typically involve metals and follow the reactivity series.
Double Displacement Reactions


Here, the positive ions of two ionic compounds swap places, leading to:
[ AB + CD \rightarrow AD + CB ]
An example could be:
[ AgNO_3 + NaCl \rightarrow AgCl + NaNO_3 ]
🧪 Note: Double displacement reactions often occur in aqueous solutions and might result in precipitate formation.
Combustion Reactions


These reactions involve the burning of a substance in oxygen, usually producing energy in the form of heat and light. The general form is:
[ CxHy + O_2 \rightarrow CO_2 + H_2O ]
For example:
[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O ]
🔥 Note: Combustion reactions typically occur when hydrocarbons or other fuels react with oxygen.
Balancing Chemical Equations

Balancing chemical equations is crucial to ensure that the law of conservation of mass is maintained. Here are steps to balance an equation:
- Write the Skeleton Equation: List the reactants and products as they appear in the reaction.
- Count Each Type of Atom: Determine the number of each type of atom on both sides of the equation.
- Use Coefficients to Balance: Adjust coefficients in front of the chemical formulas to equalize the number of atoms. Remember, the subscripts should not be changed.
- Check Your Work: Confirm that the number of atoms of each element is equal on both sides.
- Clear Extra Coefficients: Simplify coefficients if possible.
To illustrate this process, consider balancing the combustion of methane:
| Step | Action | Equation |
|---|---|---|
| 1 | Write the Skeleton Equation | \[ CH_4 + O_2 \rightarrow CO_2 + H_2O \] |
| 2 | Count Each Type of Atom | Reactants: C(1), H(4), O(2); Products: C(1), H(2), O(3) |
| 3 | Use Coefficients | \[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O \] |
| 4 | Check Your Work | Reactants: C(1), H(4), O(4); Products: C(1), H(4), O(4) |
| 5 | Clear Extra Coefficients | \[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O \] |

With this method, the equation is now balanced, adhering to the law of conservation of mass.
Wrapping Up Key Takeaways

Through understanding the five types of chemical reactions and mastering the art of balancing chemical equations, students can develop a deeper appreciation for the logic and predictability of chemistry. Each reaction type has its unique characteristics, but all share the common goal of maintaining the balance of matter in our chemical world. By applying the balancing steps outlined, one can effectively predict and understand the outcome of chemical transformations, making the study of chemistry not only scientific but also creatively engaging.
What is the purpose of balancing chemical equations?

+
The primary purpose of balancing chemical equations is to ensure that the law of conservation of mass holds, which states that matter cannot be created or destroyed in a chemical reaction, only transformed.
How can I tell if a reaction is a combustion reaction?

+
If the reaction involves the burning of a substance in oxygen (O₂), producing primarily carbon dioxide (CO₂) and water (H₂O), then it’s likely a combustion reaction.
Why do some reactions not proceed?

+
Reactions might not proceed because of energy requirements, incompatible reaction conditions, or the lack of a catalyst to lower the activation energy needed for the reaction to occur.