Discover the Answers to Types of Reactions Worksheet
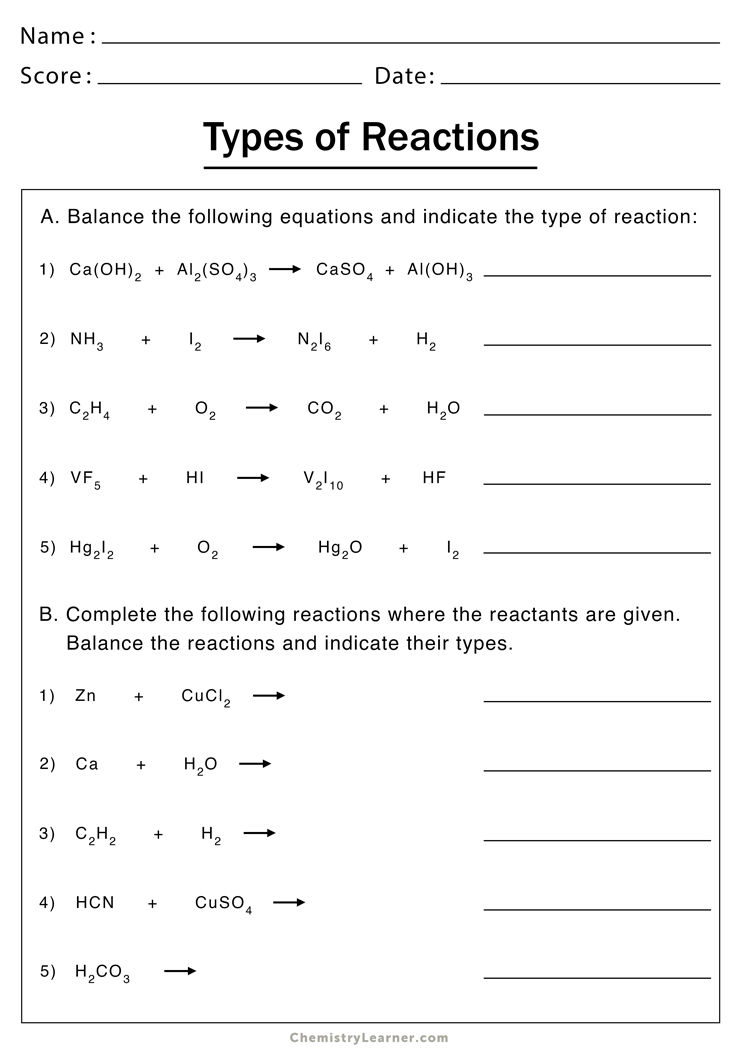
Chemical reactions are the cornerstone of chemistry, transforming substances through various processes into entirely new forms. Understanding these reactions isn't just academic; it's practical, affecting everything from the air we breathe to the products we use daily. In educational settings, a Types of Reactions Worksheet serves as an invaluable tool, helping students to differentiate and comprehend the myriad ways substances interact. This post will guide you through the typical reactions you'd find in such worksheets, offering insights into why recognizing these reactions matters, how to approach them, and what key takeaways you can expect.
Understanding Basic Reaction Types

Synthesis Reaction
In a synthesis reaction, two or more simple substances combine to form a more complex substance. The general equation for this type of reaction looks like this:
- A + B → AB
Where A and B are elements or compounds, and AB is the resulting compound. An example that often pops up in worksheets is:
- 2H2 + O2 → 2H2O (Hydrogen gas reacts with oxygen to form water)
Decomposition Reaction
Opposite to synthesis, decomposition involves breaking down a single compound into two or more simpler substances:
- AB → A + B
An example could be:
- CaCO3 → CaO + CO2 (Calcium carbonate decomposes into calcium oxide and carbon dioxide)
Single Displacement Reaction
Here, one element replaces another in a compound. The equation follows:
- A + BC → AC + B
Here's an example:
- Zn + CuSO4 → ZnSO4 + Cu (Zinc displaces copper in copper sulfate to form zinc sulfate and copper)
Double Displacement Reaction
Two compounds exchange ions, forming two new compounds. The typical equation is:
- AB + CD → AD + CB
Take this reaction, for instance:
- NaCl + AgNO3 → AgCl + NaNO3 (Sodium chloride reacts with silver nitrate to form silver chloride and sodium nitrate)
Combustion Reaction
A combustion reaction involves a substance reacting with oxygen, often resulting in heat and light. It follows:
- Fuel + O2 → CO2 + H2O + Heat
Example:
- CH4 + 2O2 → CO2 + 2H2O (Methane burns in oxygen to produce carbon dioxide and water)
Navigating the Types of Reactions Worksheet

To excel in chemical reactions worksheets, follow these strategies:
- Identify Key Indicators: Look for elements like the presence of oxygen, heat, or specific signs like color changes or precipitate formation, which are indicators of particular reactions.
- Use Balance Equations: Ensure the chemical equations are balanced. This not only ensures conservation of mass but also helps in understanding the stoichiometry of the reactions.
- Practice Recognition: Differentiate reactions by observing patterns in the reactants and products, as well as by understanding the nature of bonds and energy changes.
- Apply Knowledge: Relate theoretical knowledge to practical examples. Understanding combustion reactions, for instance, can help explain why wildfires are so difficult to control.
Why Master Chemical Reactions?

Mastering chemical reactions isn't just about passing exams. Here's why it's important:
- Environmental Impact: Understanding chemical reactions helps us comprehend pollution, like how carbon compounds released into the atmosphere contribute to global warming.
- Industrial Applications: Chemistry drives industrial processes from pharmaceuticals to metallurgy. Recognizing reaction types can streamline production, reducing costs and waste.
- Health and Safety: Knowledge of reactions is crucial in medical and emergency services, particularly in understanding how drugs interact or how to neutralize chemical spills.
Helpful Tips for Worksheet Success

Here are some practical tips for tackling a Types of Reactions Worksheet:
- Visualize Reactions: Use diagrams or illustrations to help understand how atoms rearrange in reactions.
- Check Charge Balance: Ensure that charges are balanced for ionic reactions. This helps in predicting products and understanding displacement.
- Analyze Energy Changes: Note whether a reaction is endothermic or exothermic, which can often indicate the type of reaction.
- Understand Reaction Conditions: Some reactions require specific conditions like temperature, pressure, or catalysts.
🔬 Note: Recognize that not all reactions fit neatly into one category. Some can be combinations or variations of basic types.
📝 Note: Practice makes perfect. Regularly working through different types of reactions will improve your ability to quickly identify and understand them.
In summary, understanding the different types of chemical reactions through worksheets is not only about passing a test but also about gaining a deeper insight into how our world functions on a molecular level. From synthesis and decomposition to displacement and combustion, each reaction type has its unique characteristics and implications. By identifying, balancing, and interpreting these reactions, students develop critical thinking skills applicable in various scientific and real-world scenarios. The process of mastering these reactions equips you with the knowledge to understand everyday chemical changes, from rusting to cooking, and gives you the foundation to explore further into the complexities of chemistry.
Why is it important to balance chemical equations?

+
Balancing chemical equations is crucial to conserve mass in chemical reactions. It ensures that the number of atoms of each element on the reactants’ side equals that on the products’ side, which is a fundamental principle of the law of conservation of mass.
How can I identify a synthesis reaction?

+
Identify a synthesis reaction when two or more substances combine to form a single, more complex substance. Look for the reactants being elements or simpler compounds, and the product being a compound formed from these elements.
What signs might indicate a combustion reaction?

+
Combustion reactions are typically characterized by the release of heat (exothermic), often accompanied by light (flame), and the presence of oxygen as one of the reactants. The products usually include carbon dioxide and water.



