5 Types of Chemical Reactions Worksheet Answers

In the fascinating world of chemistry, understanding how substances interact with one another is fundamental. One of the primary ways to gain this understanding is through the study of chemical reactions. Chemical reactions are the backbone of chemical change, transforming one set of substances, known as reactants, into another set called products. These reactions are categorized into different types based on the changes they undergo, each with its unique characteristics and implications. This article will delve into five common types of chemical reactions, providing you with worksheet answers to solidify your understanding of these pivotal concepts.
1. Combustion Reactions
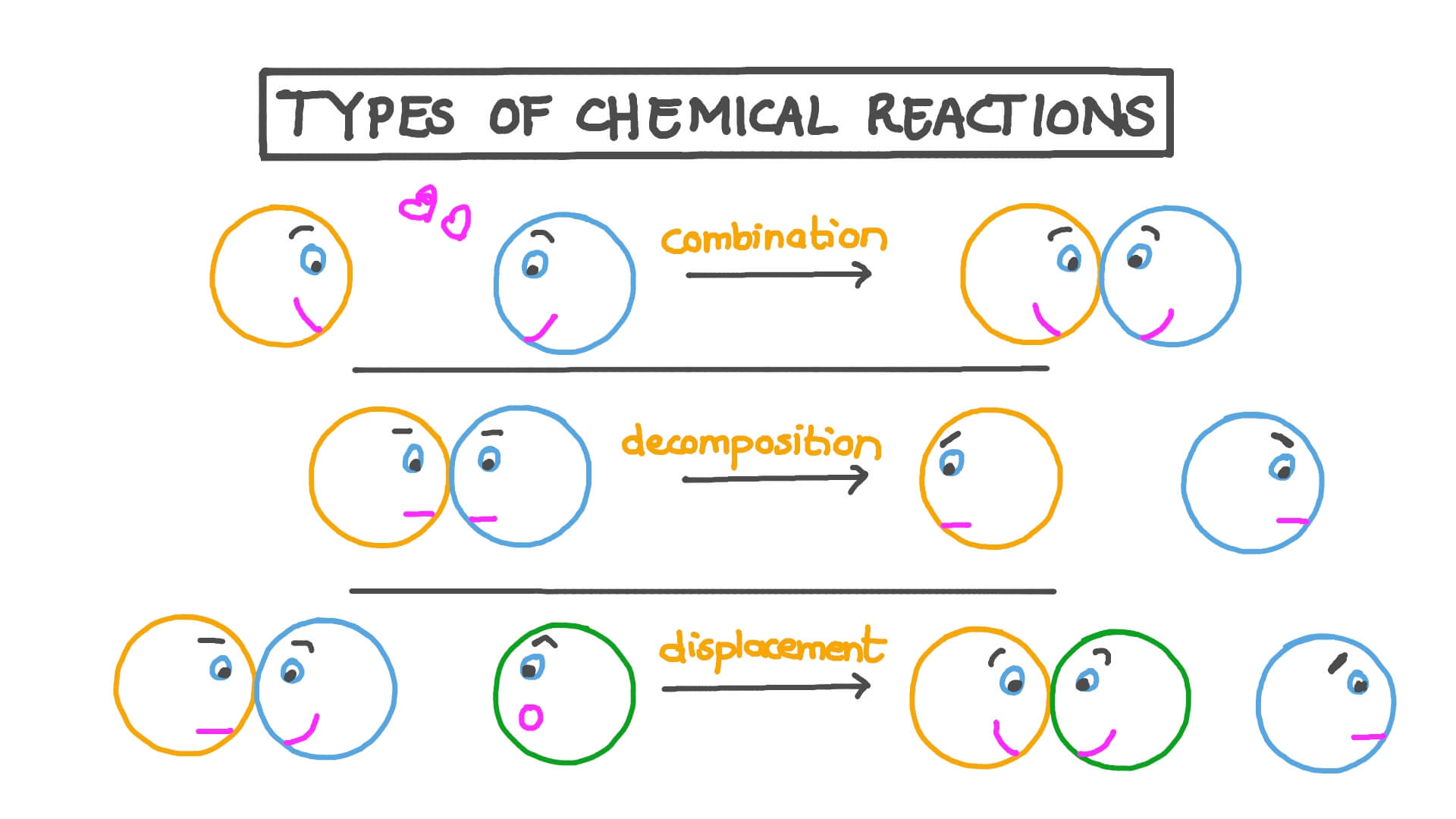
Combustion, often referred to as burning, is a high-temperature reaction between a fuel and an oxidant, typically oxygen. This reaction produces heat, light, and often CO₂ and H₂O. Here are some key points:
- The simplest form of a combustion reaction is that of hydrocarbons with oxygen, producing carbon dioxide and water:
- Example: CH₄ + 2O₂ → CO₂ + 2H₂O
- Combustion reactions can also involve other oxidants like chlorine or fluorine, but oxygen is the most common.
🔥 Note: Combustion can occur explosively if the reaction rate is rapid or as a controlled burn if managed properly.
2. Synthesis (or Combination) Reactions
Synthesis reactions involve the formation of a single product from two or more reactants. Here’s how they work:
- These reactions often produce a new compound or substance:
- Example: 2H₂ + O₂ → 2H₂O
- They are the reverse of decomposition reactions where compounds break down into simpler substances.
Synthesis reactions are fundamental in the production of fertilizers, pharmaceuticals, and in chemical synthesis labs where simple molecules are built into more complex compounds.
3. Decomposition Reactions

In contrast to synthesis, decomposition reactions involve the breakdown of a single reactant into two or more products. Here’s what you need to know:
- These reactions require energy in forms like heat or electricity:
- Example: 2H₂O₂ → 2H₂O + O₂
- Decomposition is a key process in nature, such as in the decay of organic materials.
🔍 Note: Decomposition reactions play a crucial role in environmental science, particularly in understanding how pollutants break down.
4. Single Displacement (or Single Replacement) Reactions

These reactions involve an element displacing another element from a compound. Here’s an overview:
- Typically, a more reactive element displaces a less reactive one:
- Example: Zn + CuSO₄ → ZnSO₄ + Cu
- Metal and hydrogen displacement reactions are common examples.
The activity series of metals is useful here to predict if the reaction will occur or not, making these reactions particularly interesting for predicting chemical behavior.
5. Double Displacement (or Double Replacement) Reactions
Double displacement reactions occur when two compounds exchange ions or elements with each other, forming new compounds. Here’s what makes them unique:
- These reactions often result in a precipitate, gas, or water:
- Example: AgNO₃ + NaCl → AgCl + NaNO₃
- They are common in aqueous solutions and used in processes like wastewater treatment.
📌 Note: Double displacement reactions are key to understanding solubility rules and chemical compatibility.
Understanding Chemical Reactions Through Worksheets

Worksheet exercises are excellent tools for mastering these reactions. Here is a basic table summarizing key points:
| Type of Reaction | Characteristics | Example |
|---|---|---|
| Combustion | Reacts with oxygen, produces heat/light, forms CO₂, H₂O | CH₄ + 2O₂ → CO₂ + 2H₂O |
| Synthesis | Formation of a single product | 2H₂ + O₂ → 2H₂O |
| Decomposition | Breakdown of a single reactant into multiple products | 2H₂O₂ → 2H₂O + O₂ |
| Single Displacement | An element replaces another in a compound | Zn + CuSO₄ → ZnSO₄ + Cu |
| Double Displacement | Ion or element exchange between two compounds | AgNO₃ + NaCl → AgCl + NaNO₃ |

🔬 Note: These worksheets often include problems where students identify the type of reaction, balance equations, and predict products.
To wrap up, exploring these reactions not only builds a foundational understanding of chemistry but also opens up avenues for practical applications in various industries. Combustion powers our vehicles, synthesis builds our medicines, decomposition helps us recycle materials, while displacement reactions inform us on metal reactivities and ionic behavior. Through diligent study and practice, these reactions can be mastered, offering insights into the behavior of matter at a molecular level, and propelling your scientific curiosity forward.
What are the signs of a chemical reaction?

+
Signs of a chemical reaction include the production of heat or light, color change, gas release, formation of a precipitate, and changes in odor or temperature.
How can one balance chemical equations?

+
To balance a chemical equation, ensure the number of atoms for each element is equal on both sides of the equation. Adjust coefficients in front of compounds or elements accordingly.
Why are some substances more reactive than others?

+
Reactivity depends on factors like electron affinity, ionization energy, and the electronic structure of atoms. Elements seeking to lose or gain electrons to achieve a stable electron configuration tend to be more reactive.