Mastering Chemical Reactions: Worksheet Answer Key

Learning chemistry, particularly the realm of chemical reactions, can be a daunting task for students. Understanding the intricate mechanisms, balancing equations, and recognizing reaction types are fundamental skills in mastering this subject. This comprehensive guide will not only explain various types of chemical reactions but also provide a detailed answer key for a standard chemical reactions worksheet. This aims to demystify these concepts, offering insights into the chemical ballet of matter transformation.
Understanding Chemical Reactions

At its core, a chemical reaction involves the transformation of substances, known as reactants, into new compounds, called products. Here’s how we categorize chemical reactions:
- Combination Reactions: Two or more substances combine to form a single product.
- Decomposition Reactions: A single compound breaks down into two or more simpler substances.
- Single Displacement Reactions: An element replaces another element in a compound.
- Double Displacement Reactions: Atoms or ions from two different compounds swap places, forming two new compounds.
- Combustion Reactions: Usually involve oxygen, often resulting in the production of water and carbon dioxide.
By understanding these categories, students can predict the outcomes of reactions, making the study of chemistry less abstract and more concrete.
Worksheet Answer Key
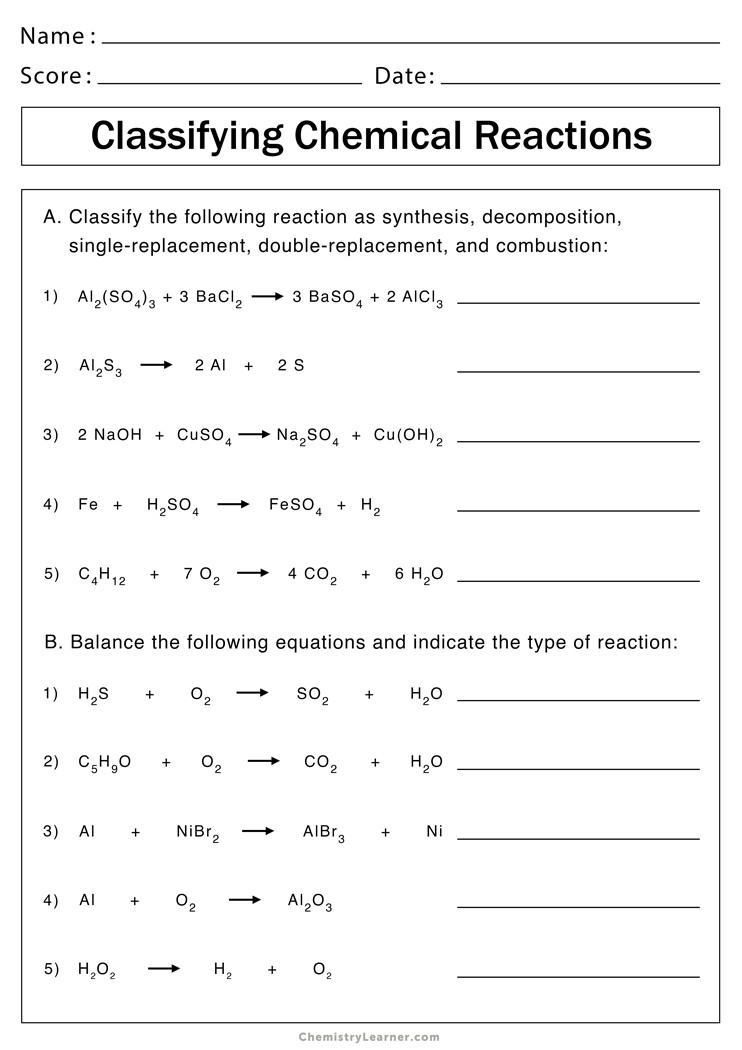
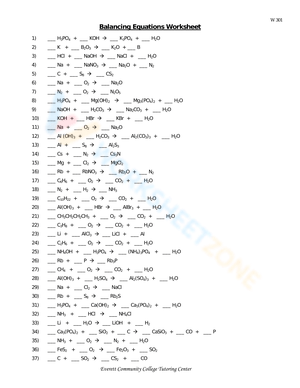
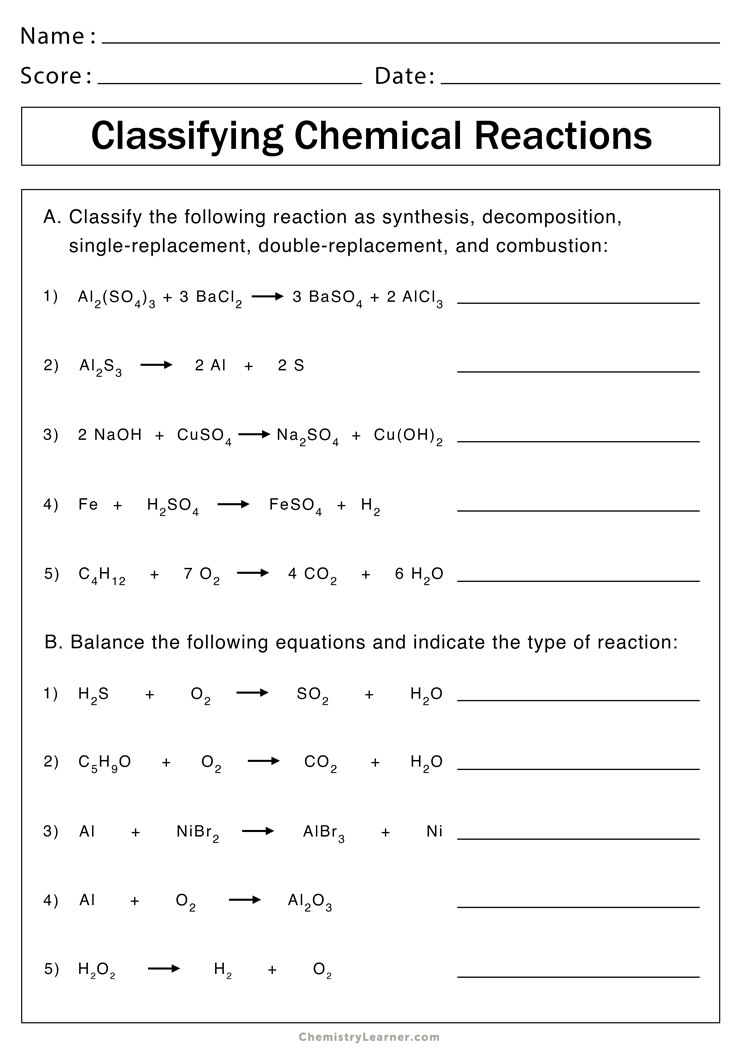
| Question Number | Type of Reaction | Reactants | Products | Balanced Equation |
|---|---|---|---|---|
| 1 | Decomposition | H2CO3 | H2O, CO2 | H2CO3 → H2O + CO2 |
| 2 | Single Displacement | Zn, CuSO4 | ZnSO4, Cu | Zn + CuSO4 → ZnSO4 + Cu |
| 3 | Combination | H2, O2 | H2O | 2H2 + O2 → 2H2O |

This table provides a clear structure for identifying reaction types, reactants, products, and the correct balanced chemical equation for each reaction, helping students master these concepts through practical application.
💡 Note: Balancing chemical equations is crucial as it respects the law of conservation of mass, ensuring the total mass of reactants equals the mass of products.
Steps to Balance Chemical Equations
- Write out the given chemical equation with reactants on the left and products on the right.
- Identify the number of atoms of each element on both sides.
- Adjust coefficients to make the number of each atom the same on both sides, starting with the most complex molecule.
- Check your work by counting atoms again. Make sure the equation is fully balanced.
- If you’ve made changes, ensure the equation remains correctly written and in its lowest terms.
🔍 Note: It's often useful to balance common elements like hydrogen and oxygen last due to their potential to be present in multiple compounds.
In this guide, we've delved into the diverse world of chemical reactions, outlined common types, and provided a structured worksheet answer key to aid in learning. The key points to take away include:
- An overview of chemical reaction categories with examples.
- Detailed steps for balancing chemical equations.
- A comprehensive worksheet answer key for practical application.
To further deepen your understanding, engage with these reactions by experimenting with various substances or through online simulation tools, which can visually demonstrate these processes.
What is a double displacement reaction?

+
A double displacement reaction occurs when parts of two different compounds swap places, forming two new compounds. This reaction often results in the formation of a solid precipitate, gas, or liquid water. An example would be the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl), yielding silver chloride (AgCl) and sodium nitrate (NaNO3).
Can chemical reactions be reversed?

+
While many reactions are irreversible under normal conditions, some chemical reactions can indeed be reversed. This is known as a reversible reaction, where the products can react to form the original reactants. Such reactions often reach a state of equilibrium where both reactants and products coexist in a stable ratio.
What is the importance of balancing chemical equations?

+
Balancing chemical equations is crucial because it reflects the law of conservation of mass. It ensures that the number of atoms of each element involved in the reaction remains constant, which is essential for understanding the stoichiometry and the efficiency of chemical reactions.
How do combustion reactions differ from others?

+
Combustion reactions involve oxygen and are characterized by the release of energy, often in the form of heat and light. Unlike other reactions, they produce compounds like water and carbon dioxide as the primary products, often leading to an exothermic process.