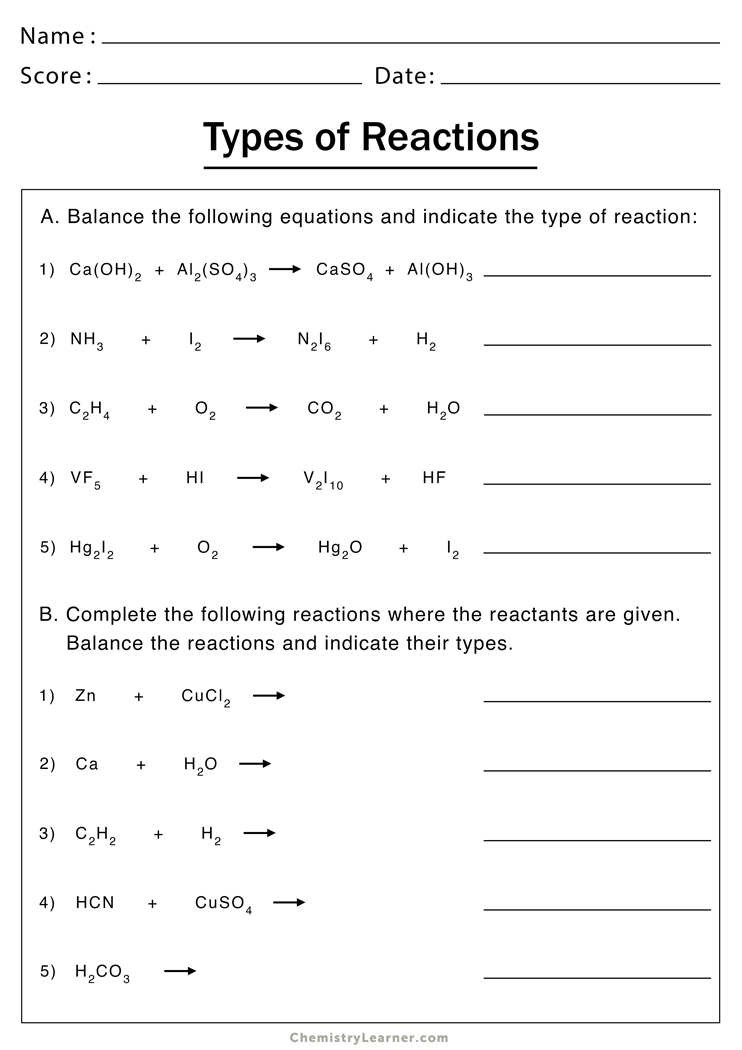
Type of Reactions Worksheet Answers: Your Chemistry Cheat Sheet
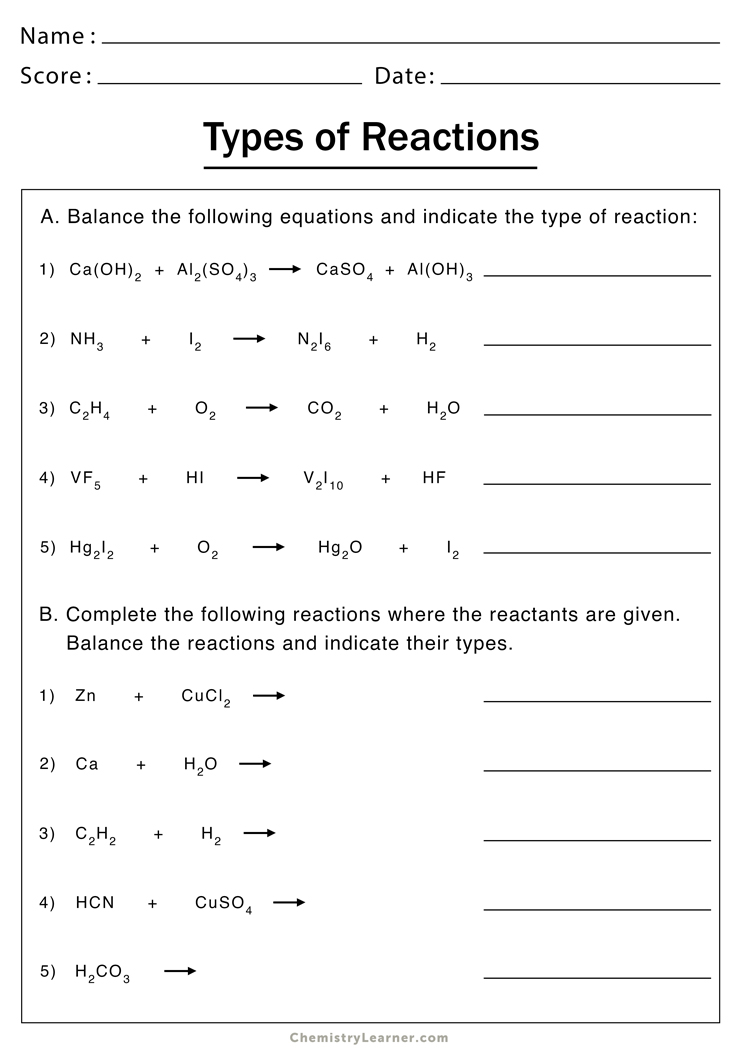
Understanding types of chemical reactions is fundamental to mastering chemistry. From the fiery combustion of fuels to the subtle precipitation that forms in a solution, chemical reactions are all around us, shaping our environment, technology, and lives. In this comprehensive guide, we will delve into the different types of reactions, discuss their characteristics, and provide answers to common worksheet questions to help students and enthusiasts alike grasp these concepts effortlessly.
Key Types of Chemical Reactions

Chemistry involves various reactions, each categorized by distinct changes. Here are the principal types of chemical reactions:
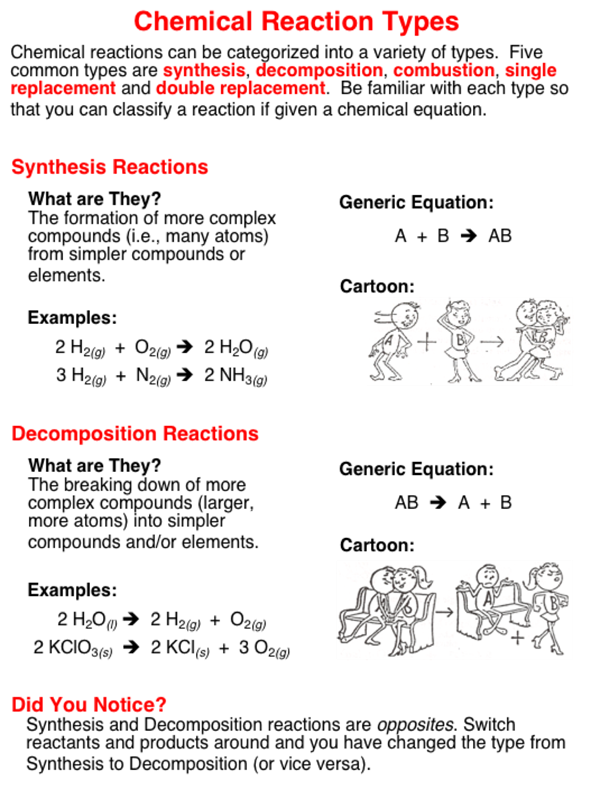
- Synthesis Reactions: Where two or more substances combine to form a new compound.
- Decomposition Reactions: A single compound breaks down into two or more simpler substances.
- Single Displacement Reactions: One element from a compound is displaced by another element.
- Double Displacement Reactions: Two compounds exchange ions or elements, leading to the formation of two new compounds.
- Acid-Base Reactions: When an acid reacts with a base, producing water and a salt.
- Combustion Reactions: A compound reacts with oxygen, often releasing energy in the form of heat or light.
- Precipitation Reactions: When two solutions combine to form an insoluble compound that settles out.
- Redox Reactions: Oxidation-reduction reactions where electrons are transferred between reactants.
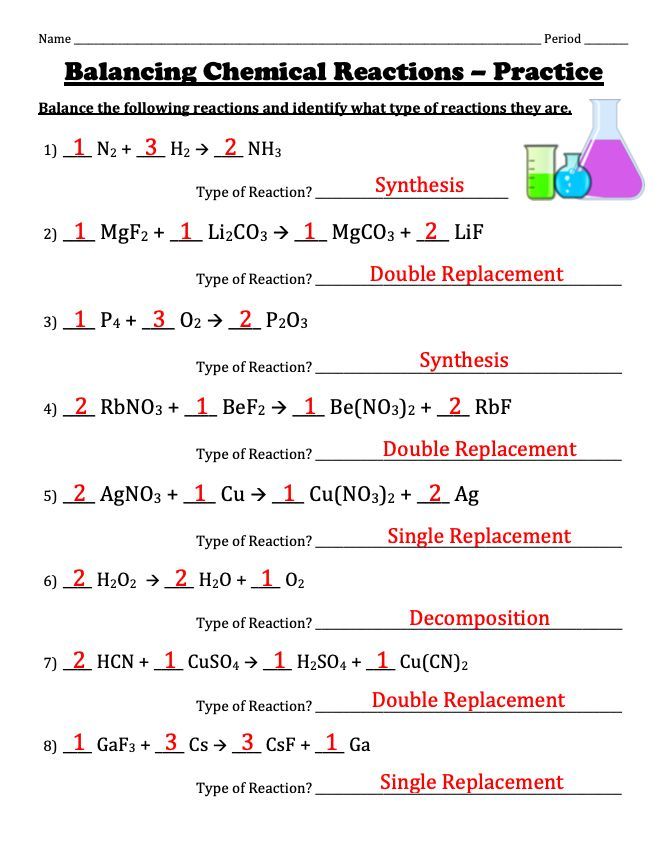
1. Synthesis Reactions

Synthesis, or combination, reactions are when two or more substances react to form a single product. The general form is:
A + B → AB
Here, 'A' and 'B' are reactants, and 'AB' is the product. Common examples include the formation of water from hydrogen and oxygen, or the synthesis of sodium chloride from sodium and chlorine:
2H₂ + O₂ → 2H₂O 2Na + Cl₂ → 2NaCl
🔬 Note: Ensure you balance the equations to maintain the law of conservation of mass.
2. Decomposition Reactions
Decomposition is the opposite of synthesis, where a compound breaks down into simpler components:
AB → A + B
A notable decomposition reaction is the thermal decomposition of calcium carbonate to produce calcium oxide and carbon dioxide:
CaCO₃ → CaO + CO₂
Decomposition often requires energy, usually in the form of heat or light.
3. Single Displacement Reactions

In single displacement reactions, one element displaces another in a compound. The reaction follows the pattern:
A + BC → AC + B
A well-known example is when zinc displaces copper in a copper sulfate solution:
Zn + CuSO₄ → ZnSO₄ + Cu
🔍 Note: The activity series helps determine whether displacement will occur.
4. Double Displacement Reactions

Two compounds exchange ions to form two new compounds. The equation looks like:
AB + CD → AD + CB
An example involves barium nitrate and sodium sulfate forming barium sulfate and sodium nitrate:
Ba(NO₃)₂ + Na₂SO₄ → BaSO₄ + 2NaNO₃
5. Acid-Base Reactions
These are also known as neutralization reactions, where an acid and a base react to produce a salt and water:
HCl + NaOH → NaCl + H₂O
⚠️ Note: Always check for net ionic equations to confirm spectator ions.
6. Combustion Reactions

Combustion involves a hydrocarbon (or other organic compounds) reacting with oxygen, typically producing carbon dioxide and water. The most straightforward combustion reaction is that of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
7. Precipitation Reactions

Here, a solid product (precipitate) forms from the interaction of two solutions. A classic example is the formation of silver chloride:
AgNO₃ + NaCl → AgCl(s) + NaNO₃
8. Redox Reactions

Redox reactions involve a change in oxidation state, where one species is oxidized (loses electrons) and another is reduced (gains electrons). A simple example is the reaction of magnesium with oxygen:
2Mg + O₂ → 2MgO
Recapitulation

Having explored these reactions, we've provided a comprehensive understanding of how chemicals interact, from synthesis to redox. Remember:
- Chemical reactions follow specific patterns.
- Knowing these patterns helps predict outcomes and balance equations.
- Practical applications of these reactions are all around us, from natural processes to industrial manufacturing.
What is the difference between synthesis and decomposition?
+
Synthesis reactions involve the formation of a compound from simpler substances, whereas decomposition reactions are about breaking a compound down into simpler substances.
Can a reaction be classified under more than one type?

+
Yes, particularly redox reactions, as they can also be combustion or single displacement reactions. However, the primary classification often prioritizes one type based on the context or the most noticeable change.
How do you balance chemical equations?
+
Balancing chemical equations involves ensuring the number of atoms of each element is the same on both sides of the equation. Start by counting the atoms of each element on both sides, adjust coefficients to make the numbers equal, and repeat the process until all atoms balance.
What are spectator ions in acid-base reactions?
+
Spectator ions are ions that do not participate in the chemical reaction. They remain unchanged and are present in the same form on both sides of the ionic equation.