Thermochemical Equations Worksheet: Master Energy Calculations Easily

Understanding Thermochemical Equations
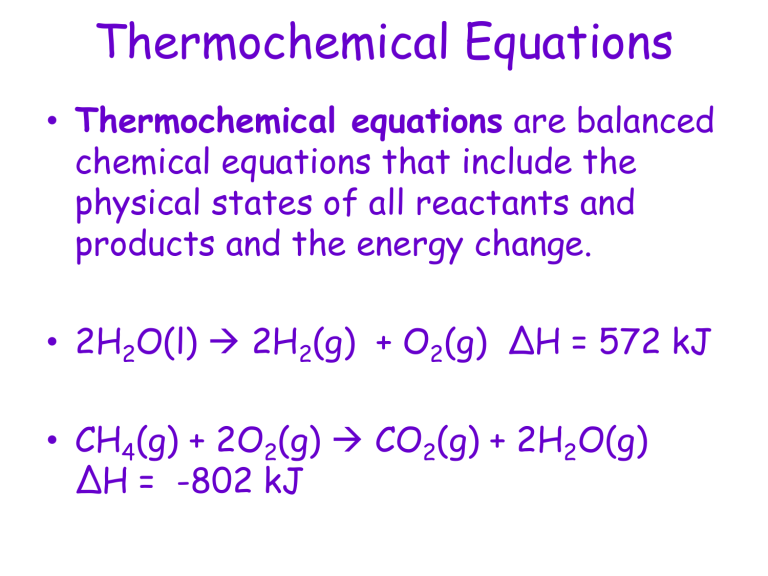
Thermochemical equations are at the heart of thermodynamics, providing a fundamental understanding of the energy changes that occur during chemical reactions. These equations not only describe the stoichiometry of reactants and products but also the heat absorbed or released, known as the enthalpy change (ΔH). Mastering thermochemical equations is essential for chemists, students, and anyone interested in the practical applications of chemical energy.
The Basics of Thermochemical Equations

A thermochemical equation includes the chemical reaction along with the associated energy change. Here's what you need to know:
- Chemical Equation: It shows the balanced equation with the states of matter (s, l, g, aq).
- Enthalpy Change (ΔH): The energy change for the reaction. If ΔH is positive, the reaction is endothermic, absorbing heat from the surroundings. If ΔH is negative, it's exothermic, releasing heat.
- Units: Enthalpy change is typically measured in kJ or kJ/mol.
Writing Thermochemical Equations

To write a thermochemical equation:
- Write down the chemical reaction.
- Include the states of matter for each substance.
- Indicate the enthalpy change either on the left or right side of the equation, or separate it by a comma or arrow.
- Remember to maintain the stoichiometry when adjusting the amounts of reactants or products; the enthalpy change must be scaled proportionally.
🔬 Note: Ensure your equation balances both mass and charge as well as energy.
Using Thermochemical Equations for Energy Calculations

Here are some critical applications of thermochemical equations:
Calculating Heat of Reaction

To determine the heat released or absorbed during a reaction, use the following steps:
- Write the thermochemical equation.
- Determine the moles of a reactant or product involved.
- Use the stoichiometry of the equation to calculate the heat change based on ΔH.
Example:
2H2(g) + O2(g) → 2H2O(g), ΔH = -483.6 kJ If you combust 1.5 moles of hydrogen, the energy released would be: Heat = (-483.6 kJ / 2 mol H2) × 1.5 mol H2 = -362.7 kJ
Reactions in Standard States

Standard enthalpy changes (ΔH°) are measured at standard conditions (298 K and 1 atm). This allows for a more consistent comparison of energy changes:
- ΔH°: Enthalpy change under standard conditions.
- Formation: The energy change when one mole of a compound is formed from its elements in their standard states.
- Combustion: The heat of combustion of a substance, the energy released when one mole of the substance is completely burned in oxygen.
Hess's Law

Hess's Law is particularly useful when measuring the enthalpy change of a reaction indirectly:
- Summarize enthalpies of reactions to get the overall enthalpy change for a series of reactions leading to the desired reaction.
- The total enthalpy change is the sum of the enthalpy changes of the individual steps.
Example:
C(s) + O2(g) → CO2(g), ΔH° = -393.5 kJ 2CO(g) + O2(g) → 2CO2(g), ΔH° = -566 kJ To find ΔH° for C(s) + 1/2 O2(g) → CO(g): ΔH° = -393.5 kJ + (1/2)(-566 kJ) = -393.5 kJ - 283 kJ = -676.5 kJ
Notes on Manipulating Thermochemical Equations

- Reversing the Equation: If you reverse the direction of a thermochemical equation, the sign of ΔH changes.
- Multiplying: If you multiply the entire equation by a factor, multiply ΔH by the same factor.
- Addition: When adding two thermochemical equations, simply add their ΔH values.
💡 Note: Be careful with signs and coefficients to maintain energy conservation.
Final Thoughts

Thermochemical equations are not just mathematical constructs; they represent real physical phenomena. Whether you're calculating the heat of combustion or exploring energy transformations, understanding how to write, manipulate, and use these equations will enhance your grasp of chemistry. They reveal the energy content of substances, the energy released or absorbed during reactions, and are fundamental in applications ranging from fuel efficiency to industrial processes. By mastering thermochemical equations, you're not just learning equations; you're unlocking the energy secrets of the universe, one reaction at a time.
What is the difference between an endothermic and an exothermic reaction?

+
An endothermic reaction absorbs heat from the surroundings (positive ΔH), while an exothermic reaction releases heat to the surroundings (negative ΔH).
How can I tell if a reaction is at standard conditions?

+
A reaction is at standard conditions if it occurs at 298 K (25°C) and 1 atm pressure, and all substances are in their standard states (pure form at 1 atm).
Why is Hess’s Law important?

+
Hess’s Law is crucial because it allows you to determine the enthalpy change of a reaction indirectly by summing the enthalpy changes of known reactions.