Maximize Chemistry Success with Yield Calculations Guide

Welcome to our comprehensive guide on maximizing success in chemistry through yield calculations! Understanding yield calculations is crucial for students and professionals alike, as it directly impacts the efficiency and cost-effectiveness of chemical reactions. Whether you are synthesizing compounds or conducting laboratory experiments, mastering these calculations can significantly enhance your ability to optimize processes and achieve the best possible results.
What is Yield in Chemistry?

Yield in chemistry refers to the amount of product obtained from a chemical reaction compared to the maximum theoretical amount possible. There are several types of yield to consider:
- Theoretical Yield - The maximum amount of product that could be formed from the reactants based on stoichiometric calculations.
- Actual Yield - The quantity of product actually obtained from the reaction.
- Percent Yield - The ratio of the actual yield to the theoretical yield, expressed as a percentage.
📝 Note: The percent yield is always less than or equal to 100% due to various inefficiencies in the reaction process.
Calculating Yield

To calculate yield in a chemical reaction:
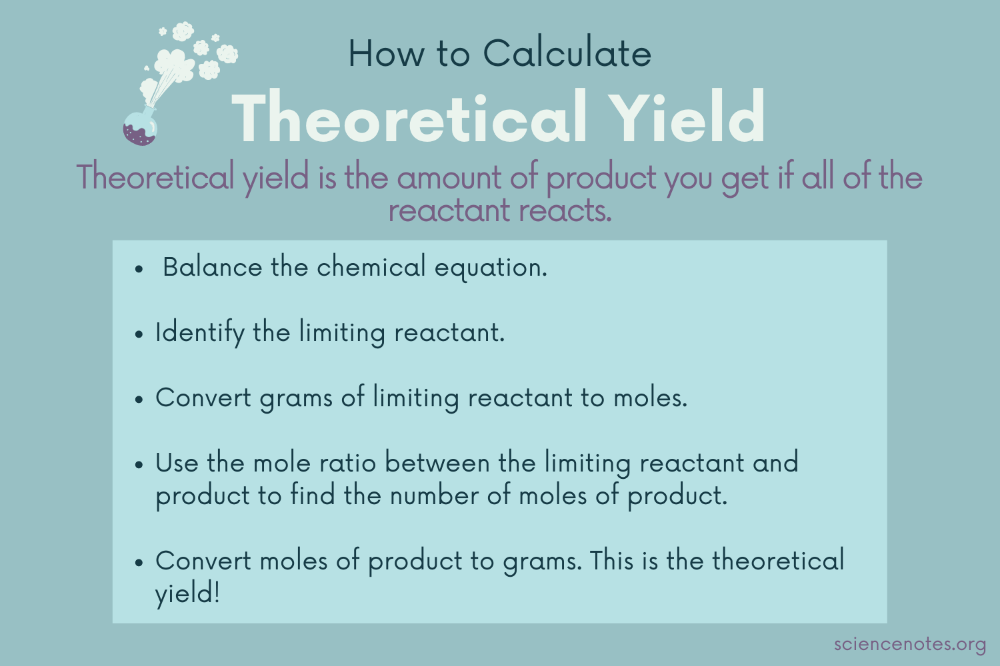
1. Determine Theoretical Yield

The theoretical yield is calculated using the stoichiometry of the balanced chemical equation. Here’s how:
- Balance the equation to find the moles of each reactant.
- Use the limiting reagent to calculate how many moles of product would be produced.
- Convert moles of product to mass using the molar mass.
🔍 Note: The limiting reagent is the reactant that is completely consumed first, limiting the amount of product formed.
2. Measure Actual Yield

The actual yield is the mass of the product you isolate after performing the reaction. It’s usually less than the theoretical yield due to:
- Incomplete reaction
- Side reactions
- Product loss during purification
3. Calculate Percent Yield

Now, calculate the percent yield using the formula:
Optimizing Chemical Yields

Here are some strategies to optimize your chemical reactions for better yields:
Choosing Reactants Wisely
Selecting high-quality reactants:
- Ensure purity to reduce side reactions.
- Use an excess of the limiting reagent to push the reaction forward.
Reaction Conditions

Adjusting reaction conditions can lead to improved yields:
- Temperature - Heat or cool the reaction to find the optimal temperature.
- Pressure - Adjust pressure for gas-phase reactions.
- Concentration - Dilution or concentration can impact the rate of reaction.
Minimizing Losses

To minimize product loss:
- Use efficient separation techniques.
- Handle products with care during transfer or isolation.
🔹 Note: Vacuum filtration or centrifugation can often yield more product than other methods like gravity filtration.
Applications of Yield Calculations

Yield calculations are not just theoretical exercises; they have real-world applications:
In Industry

Industrially, maximizing yield is crucial for:
- Cost reduction through less waste.
- Increased efficiency in resource utilization.
In Education

Yield calculations teach students about:
- Stoichiometry and chemical reactions.
- Practical skills in lab work.
Challenges and Solutions

Understanding the common issues and finding solutions is key:
Common Issues

- Side Reactions: Unwanted reactions can occur, reducing yield.
- Reagent Purity: Impure reagents lead to lower yields.
- Product Loss: Loss during purification or transfer.
Solutions

- Selective Catalysts: Use catalysts to steer reactions toward desired products.
- Protecting Groups: Employ protecting groups to mask reactive sites.
- Careful Handling: Implement careful handling and efficient recovery techniques.
At the end of the day, your ability to maximize chemical yields depends on a combination of theoretical knowledge, practical skills, and a keen understanding of the specific chemistry involved in each reaction. By systematically addressing each step from reagent selection to the purification of products, you can significantly increase your success rate in chemistry experiments and applications.
Why is the actual yield usually less than the theoretical yield?
+The actual yield is usually less than the theoretical yield due to inefficiencies in the reaction process such as incomplete reactions, side reactions, losses during product isolation, and reagent impurities.
How can reaction conditions affect yield?
+Reaction conditions like temperature, pressure, and concentration can significantly impact the yield by influencing the reaction kinetics, the extent of the reaction, and the formation of by-products.
Can yield be improved with additional techniques?
+Yes, techniques like using selective catalysts, employing protecting groups, and optimizing separation methods can improve yield by minimizing side reactions and product loss.