5 Essential Mole Calculations for Chemistry Mastery

Learning mole calculations is indispensable for anyone studying chemistry. These calculations help you navigate the atomic and molecular world, allowing you to understand chemical reactions on a microscopic level. Here's a guide on five essential mole calculations every chemistry student should master.
The Basics: Understanding the Mole Concept
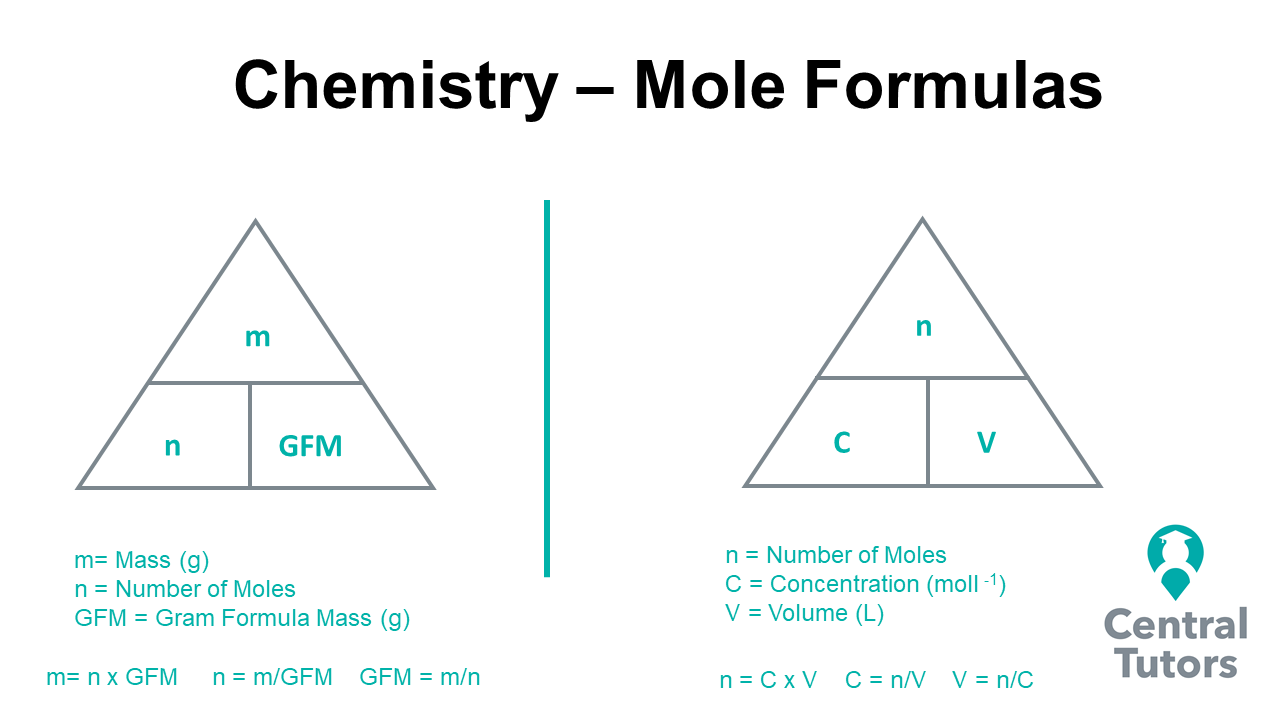
Definition: A mole is the SI unit for amount of substance, denoted by ‘mol’. One mole represents Avogadro’s number of particles, which is approximately 6.022 x 1023. Whether those particles are atoms, ions, or molecules, a mole always contains this immense number.
🔬 Note: Avogadro's number was named after the Italian scientist Amedeo Avogadro and serves as a bridge between the microscopic world of atoms and the macroscopic world of lab-scale quantities.
Calculating Molar Mass

To perform mole calculations, you need to know the molar mass of substances involved in a reaction. Here’s how:
- Find the Atomic Mass: Use the periodic table to locate the atomic mass of each element involved. Remember, atomic mass is the average mass of all the isotopes of an element.
- Sum for Molecules: If you’re dealing with compounds or molecules, sum the atomic masses of all the constituent elements, considering their subscript numbers for molecular formulas.
Example:
| Element | Number of Atoms | Atomic Mass (g/mol) | Total Mass (g/mol) |
|---|---|---|---|
| H (Hydrogen) | 1 | 1.008 | 1.008 |
| O (Oxygen) | 1 | 16.00 | 16.00 |
| Water (H2O) | 18.016 | ||
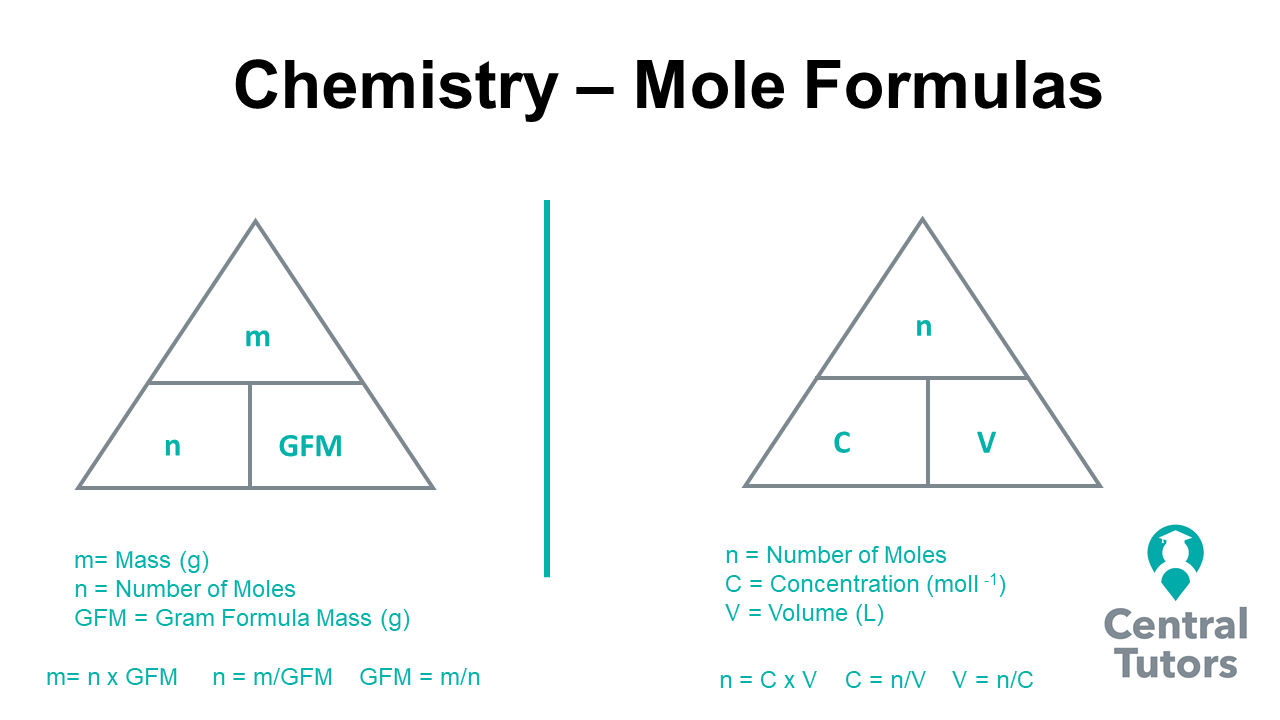
Molarity Calculations

Molarity (M) is the concentration of a solution expressed as moles of solute per liter of solution. Here’s the formula:
[ M = \frac{\text{Moles of Solute}}{\text{Liters of Solution}} ]- Determine Moles: Use the given mass or volume of the solute along with its molar mass to find moles.
- Find Volume: Use the volume of the solution (after solute and solvent are mixed), usually in liters.
Stoichiometry
Stoichiometry deals with the quantitative relationships in chemical reactions. The essence here is to use the balanced chemical equation to predict how much of each reactant or product is involved.
- Write the Balanced Equation: This is key for stoichiometry calculations.
- Convert to Moles: Use molar mass to convert given masses to moles.
- Use Mole Ratios: The coefficients in the balanced equation tell you the mole ratio of reactants and products.
Mass to Mass Calculations
These calculations involve converting the mass of a reactant to the mass of a product. Here’s the process:
- Convert Mass to Moles: Use molar mass to get moles of the reactant.
- Apply Stoichiometry: Use the balanced equation to find the moles of the product.
- Convert Moles to Mass: Use the molar mass of the product to find the mass produced.
In mastering mole calculations, the key is understanding the underlying principles: the conservation of mass and the law of definite proportions. By applying these, chemistry students can predict outcomes of reactions, understand the chemistry behind biological processes, and ensure chemical processes are carried out efficiently.
Throughout your journey in chemistry, these five essential mole calculations will serve as the foundation for understanding chemical reactions and stoichiometry. Whether you're dealing with large-scale industrial processes or minute biochemical reactions, these calculations allow for precision and control, making them invaluable for any aspiring chemist or scientist.
Why is Avogadro’s number significant in chemistry?

+
Avogadro’s number allows us to relate the microscopic world of atoms and molecules to macroscopic measurements. It’s the bridge between the quantity of atoms in a substance and the mass of that substance, which is crucial for stoichiometry and understanding the scale of reactions.
How can I remember the molar mass of elements?
+
Use mnemonic devices or remember that the molar mass is close to the atomic number of elements for simplicity. For more precise work, always refer to the periodic table, which lists the most accurate atomic masses.
What’s the difference between moles and molarity?

+
Moles refer to the amount of substance, while molarity is a measure of the concentration of a solution. Moles are dimensionless; molarity includes volume and is expressed in moles per liter (mol/L).