5 Essential Tips for Mole Chemistry Calculations

Mastering mole chemistry is crucial for any chemistry student as it forms the backbone of many chemical calculations. Whether you're calculating the mass of a substance, determining the number of particles, or balancing chemical equations, understanding moles is indispensable. Here are five essential tips that will not only help you master mole calculations but also make your learning journey smoother and more effective.
1. Understand the Concept of the Mole

Before diving into calculations, grasp what a mole represents. The mole, symbolized as mol, is defined as the amount of substance that contains as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in exactly 12 grams of carbon-12. This number, known as Avogadro’s number, is approximately 6.022 × 1023.
To internalize this concept:
- Think of a mole as a 'chemist's dozen' where one dozen equals 12, but here, one mole equals Avogadro's number.
- Remember that moles are a convenient way to count particles in chemistry, making calculations simpler.
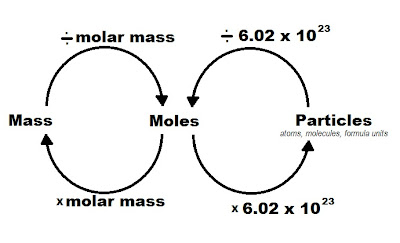
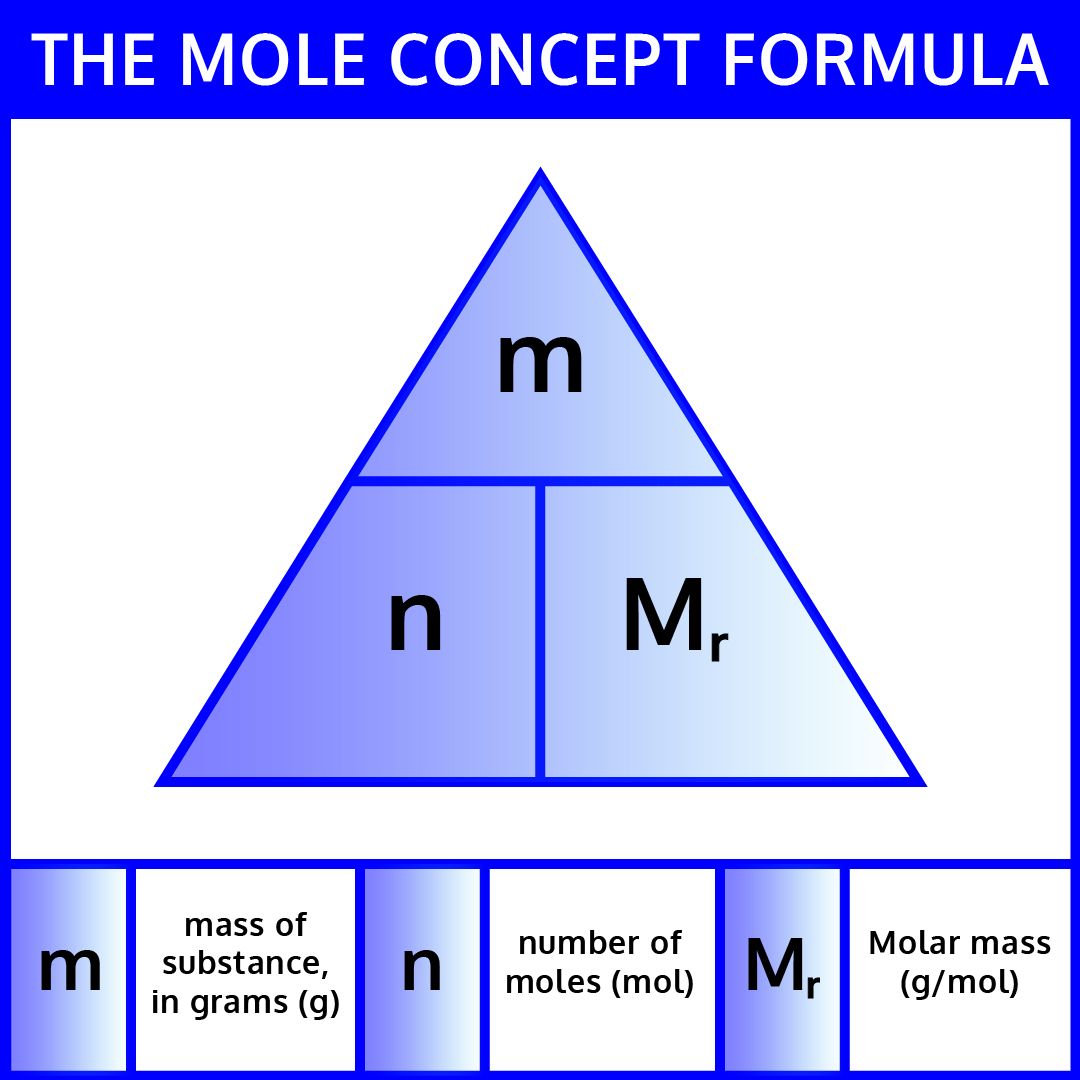
2. Use Molar Mass to Convert Between Grams and Moles

Molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol). Here's how to use it:
- Convert grams to moles: Use the formula Moles = Mass (g) / Molar Mass (g/mol).
- Convert moles to grams: Simply multiply the number of moles by the molar mass.

⚙️ Note: Always check your periodic table for the most accurate atomic masses when calculating molar masses.
3. Master Mole Ratios for Chemical Reactions

Balancing chemical equations involves using mole ratios to ensure the law of conservation of mass is not violated:
- Write the balanced chemical equation.
- Identify the mole ratio between reactants and products.
- Use these ratios to convert between moles of different substances in a reaction.
| Compound | Molar Ratio |
|---|---|
| N2 | 1 |
| H2 | 3 |
| NH3 | 2 |

🔧 Note: For complex reactions, double-check the coefficients to avoid errors in mole calculations.
4. Employ Limiting Reactants in Calculations

In most reactions, one reactant will be completely consumed before others, known as the limiting reactant. Here’s how to find it:
- Calculate the moles of each reactant available.
- Determine the moles of product each reactant can produce using the mole ratio.
- The reactant producing the least amount of product is the limiting reactant.
5. Practice Dimensional Analysis

Dimensional analysis, or the factor-label method, simplifies conversion between different units by using conversion factors:
- Identify what unit you need to end up with.
- Set up the conversion factors that will cancel out the units you don’t want.
- Multiply through the conversion factors to get to your desired unit.
📚 Note: Practice setting up problems with different units and conversion factors to become proficient in dimensional analysis.
In summary, mastering mole chemistry requires a foundational understanding of what a mole represents, how to convert between moles and mass, the significance of mole ratios in chemical reactions, identifying limiting reactants, and utilizing dimensional analysis for unit conversions. These tips not only simplify complex calculations but also ensure accuracy in your chemical analysis. With regular practice and application of these strategies, you'll find mole chemistry calculations become second nature.
What is Avogadro’s number?

+
Avogadro’s number is approximately 6.022 × 1023, which is the number of particles (atoms, molecules, ions, etc.) in one mole of a substance.
How do you calculate the limiting reactant in a chemical reaction?

+
To find the limiting reactant, calculate the moles of each reactant available, then determine how much product each can produce based on the mole ratio. The reactant that produces the least amount of product is the limiting reactant.
Why is it important to balance chemical equations?
+
Balancing chemical equations ensures that the law of conservation of mass is adhered to, meaning the number of atoms of each element must be the same on both sides of the equation.
What is the purpose of using molar mass?
+
Molar mass is used to convert between the mass of a substance and the number of moles, enabling chemists to easily count atoms or molecules in a sample by weighing it.
Can dimensional analysis be used in non-chemistry fields?
+
Yes, dimensional analysis is a versatile method used in physics, engineering, and other fields for converting between different units of measurement.