5 Essential Facts About the History of the Atom

Understanding the atom is essential for grasping modern physics, chemistry, and an array of scientific principles that govern the universe. The history of the atom's discovery and conceptualization spans centuries and involves contributions from many brilliant minds. Here are five key facts that shed light on the evolution of atomic theory.
1. Early Atomistic Theory - Ancient Wisdom
The idea of the atom can be traced back to ancient Greece, where philosophers like Leucippus and Democritus first proposed the concept of atomos—meaning indivisible—around the 5th century BCE. They theorized that matter was composed of tiny, indivisible parts that were eternal, unchangeable, and moved in an infinite void. Here’s a brief look at their views:
- Materialism: Everything is made of atoms and void.
- Qualities of Atoms: Atoms vary in shape, size, and arrangement, but are indestructible.
2. Dalton’s Modern Atomic Theory - The Scientific Revolution

Fast forward to the early 19th century, John Dalton reintroduced the atomic idea with a modern scientific twist. His atomic theory, formulated in 1803, provided a framework for understanding chemical reactions based on the following principles:
- All matter consists of indivisible particles called atoms.
- Atoms of the same element are identical in mass and properties.
- Compounds are formed by the combination of two or more different atoms in fixed, whole-number ratios.
📝 Note: Dalton’s theory was foundational but lacked understanding of isotopes and subatomic particles, which were later discovered.
3. The Discovery of Subatomic Particles

By the late 19th and early 20th centuries, scientists began uncovering the subatomic structure of atoms. Here’s a timeline of key discoveries:
| Year | Scientist | Discovery |
|---|---|---|
| 1897 | J.J. Thomson | Electron - establishing that atoms contain negatively charged particles. |
| 1909 | Ernest Rutherford | Nucleus - showing that the atom’s mass is concentrated in a small, dense center. |
| 1919 | Ernest Rutherford | Proton - positively charged particle within the nucleus. |
| 1932 | James Chadwick | Neutron - a neutral particle within the nucleus. |

4. Quantum Mechanics and Atomic Structure
Quantum mechanics revolutionized the understanding of atoms. It provided explanations for phenomena like the atomic spectra, electron configurations, and the quantized nature of energy levels within atoms. Here are some pivotal developments:
- Bohr Model: In 1913, Niels Bohr proposed the Bohr model, suggesting electrons orbit the nucleus at specific energy levels, jumping between levels when absorbing or emitting energy.
- Wave-Particle Duality: Louis de Broglie’s wave-particle duality in 1924 suggested that particles like electrons exhibit both wave and particle properties.
- Heisenberg’s Uncertainty Principle: Werner Heisenberg introduced the idea that we can’t precisely know both an electron’s position and momentum simultaneously.
🧬 Note: Quantum mechanics provided a more accurate model of atomic behavior, replacing the simplistic Newtonian mechanics with a probabilistic approach.
5. The Standard Model and Beyond

The latter half of the 20th century and early 21st century have seen profound advancements in our understanding of particle physics. The Standard Model, developed in the late 1960s to the early 1970s, describes fundamental particles and the forces between them:
- Quarks: Building blocks of protons and neutrons, classified as up, down, charm, strange, top, and bottom.
- Leptons: Including the electron, muon, tau, and their associated neutrinos.
- Bosons: Force carriers like the photon (electromagnetism), gluon (strong nuclear force), W and Z bosons (weak nuclear force), and the Higgs boson, which imparts mass.
The discovery of the Higgs boson in 2012 confirmed the existence of the mechanism by which particles gain mass, closing a significant chapter in particle physics, yet opening up new questions about the universe’s fundamental nature.
In summary, the history of the atom reveals a fascinating journey from philosophical speculation to a rigorous scientific pursuit. The atomic theory has evolved from the indivisible particle of ancient Greece to complex structures involving subatomic particles, quantum mechanics, and the Standard Model. This development reflects humanity's relentless quest to understand the microscopic foundations of the material world. The story of the atom continues, with ongoing research into fundamental particles, dark matter, and the underlying principles of the universe.
What is the difference between an atom and a molecule?
+
An atom is the smallest unit of an element that retains the chemical properties of that element. A molecule, on the other hand, is formed when two or more atoms are chemically bonded together. For example, O₂ is a molecule composed of two oxygen atoms.
Why don’t we notice subatomic particles in our daily life?

+
Subatomic particles like electrons, protons, and neutrons are incredibly small, operating on scales that are beyond our ordinary sensory perceptions. Furthermore, their behavior is governed by quantum mechanics, which does not translate into phenomena we typically observe in everyday life.
What role did the discovery of isotopes play in Dalton’s atomic theory?

+
Dalton’s theory posited that all atoms of a given element were identical. The discovery of isotopes showed that atoms of the same element could have different masses due to the varying number of neutrons. This finding modified the understanding of atomic structure and properties.
How has the atomic model evolved since Bohr’s model?
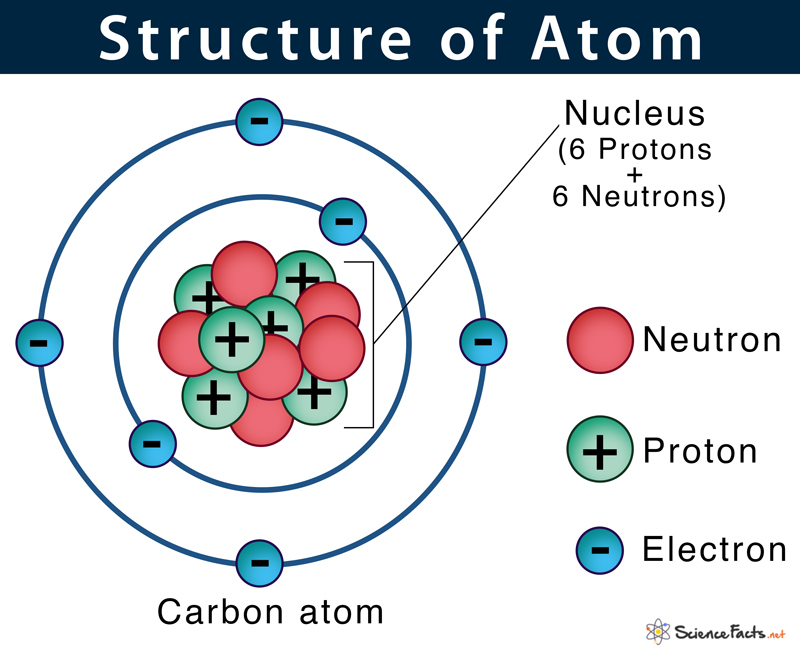
+
Since Bohr’s model, which treated electrons as particles orbiting the nucleus in fixed energy levels, the model has evolved to incorporate wave functions in quantum mechanics. The Schrödinger equation now describes electron probability clouds rather than fixed orbits, providing a probabilistic view of atomic electron configurations.
What future research might reveal about the atom?

+
Future research might delve into the fundamental nature of particles, the Higgs mechanism, dark matter, and potentially unify quantum mechanics with general relativity. The quest for a ‘theory of everything’ could provide new insights into the atom’s structure and the laws of nature at the smallest scales.