5 Essential Stoichiometry Worksheet Answers Revealed

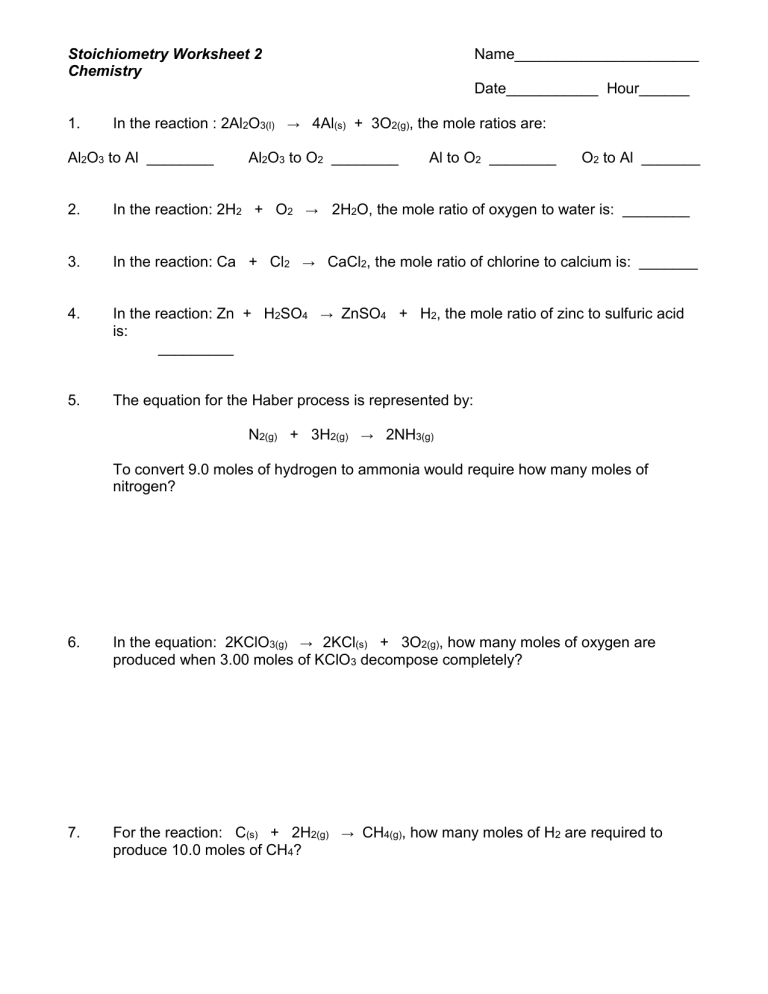
Chemistry, as a science subject, is often seen as daunting due to its intricate theories and complex calculations, but with the proper guidance, it can be made accessible and interesting. One of the foundational concepts students encounter in chemistry is stoichiometry. This branch of chemistry deals with the quantitative relationships between reactants and products in a chemical reaction. Understanding stoichiometry is crucial for students aiming to excel in chemistry, and for that purpose, we'll be delving into some key stoichiometry worksheet answers.
1. Understanding Stoichiometry Through Worksheet Answers


Stoichiometry worksheets often provide a structured way for students to practice calculations and deepen their understanding. Here are five essential stoichiometry worksheet answers that can aid in mastering the concept:
Mole to Mole Conversion

A common stoichiometry problem involves converting from moles of one substance to moles of another using a balanced chemical equation.
- Given the reaction: N₂ + 3H₂ → 2NH₃
- Question: How many moles of NH₃ can be produced from 5 moles of N₂?
- Answer:
Step Calculation Moles of N₂ 5 moles Mole Ratio From the equation, 1 mole of N₂ produces 2 moles of NH₃ Moles of NH₃ 5 moles N₂ × (2 moles NH₃ / 1 mole N₂) = 10 moles of NH₃ 
Mass to Mole Conversion

This type of problem converts the mass of a reactant or product to the number of moles.
- Given the reaction: 2Al + 3Br₂ → 2AlBr₃
- Question: How many moles of Br₂ are needed to react with 13.5g of Al?
- Answer:
- Molar mass of Al = 26.98 g/mol
- Moles of Al = Mass / Molar mass = 13.5 g / 26.98 g/mol = 0.50 mol
- Mole Ratio (Al : Br₂) = 2 : 3
- Moles of Br₂ = Moles of Al × (3⁄2) = 0.50 mol × (3⁄2) = 0.75 mol
⚠️ Note: Ensure all calculations are based on the balanced chemical equation and the stoichiometric coefficients provided therein.
Mole to Mass Conversion

Here, students are asked to find the mass of a product or reactant from a given number of moles.
- Given the reaction: 4NH₃ + 5O₂ → 4NO + 6H₂O
- Question: What is the mass of NO produced from 2 moles of NH₃?
- Answer:
- Mole Ratio (NH₃ : NO) = 1 : 1
- Moles of NO = Moles of NH₃ = 2 mol
- Molar mass of NO = 30.01 g/mol
- Mass of NO = Moles × Molar mass = 2 mol × 30.01 g/mol = 60.02 grams
Limiting Reagent Determination

Identifying which reactant will run out first is crucial for stoichiometric calculations.
- Given the reaction: 3A + 2B → C
- Question: Determine the limiting reagent for 6 moles of A and 5 moles of B.
- Answer:
- Moles of B required if A is the limiting reagent = (6 moles A) × (2 moles B / 3 moles A) = 4 moles of B
- Since we have only 5 moles of B, B will run out before A, making B the limiting reagent.
Percent Yield Calculation
This involves calculating how efficient a reaction is by comparing the actual yield to the theoretical yield.
- Given the reaction: 2CuO + C → 2Cu + CO₂
- Question: If 10.0 grams of CuO react with excess carbon, yielding 7.5 grams of Cu, what is the percent yield?
- Answer:
- Calculate theoretical yield:
- Molar mass of CuO = 79.55 g/mol
- Moles of CuO = 10.0 g / 79.55 g/mol = 0.126 mol
- Mole Ratio (CuO : Cu) = 2 : 2, so moles of Cu = moles of CuO = 0.126 mol
- Molar mass of Cu = 63.55 g/mol
- Theoretical mass of Cu = Moles × Molar mass = 0.126 mol × 63.55 g/mol = 7.977 grams
- Percent Yield = (Actual yield / Theoretical yield) × 100%
- Percent Yield = (7.5 g / 7.977 g) × 100% ≈ 94.03%
- Calculate theoretical yield:
By going through these detailed stoichiometry worksheet answers, students can understand how to apply chemical principles in a practical context, enhancing both their problem-solving skills and their grasp of stoichiometry. This foundational knowledge is pivotal for success in advanced chemistry courses and laboratory work where real-world applications come into play.
To conclude, stoichiometry is not just a theoretical concept; it's a gateway to understanding the quantitative aspects of chemical reactions. Through mastering these calculations, students are equipped to handle complex reactions, predict outcomes, and analyze experimental results with confidence.
Why are stoichiometry calculations important in chemistry?

+
Stoichiometry allows chemists to predict how much product will be produced by a given amount of reactants, optimize reactions, determine limiting reagents, and calculate reaction yields, which are essential in both industrial and academic settings.
Can stoichiometry help in real-world chemical engineering?

+
Absolutely, stoichiometry is used in chemical engineering to design reactors, analyze reaction efficiencies, optimize chemical processes, and ensure that resources are used cost-effectively.
What happens if you use an unbalanced equation in stoichiometry?

+
Using an unbalanced equation will lead to incorrect calculations because it does not reflect the true stoichiometric ratios of reactants and products. This can cause significant errors in predicting yields and understanding reaction behavior.
How can you check your stoichiometry calculations?

+
One way is to ensure that your mass or mole balances; the law of conservation of mass states that the total mass before and after a chemical reaction should be the same. Also, double-check your units, use dimensional analysis, and confirm that your calculations are based on a balanced equation.