5 Steps to Master Stoichiometry: Worksheet Key Revealed
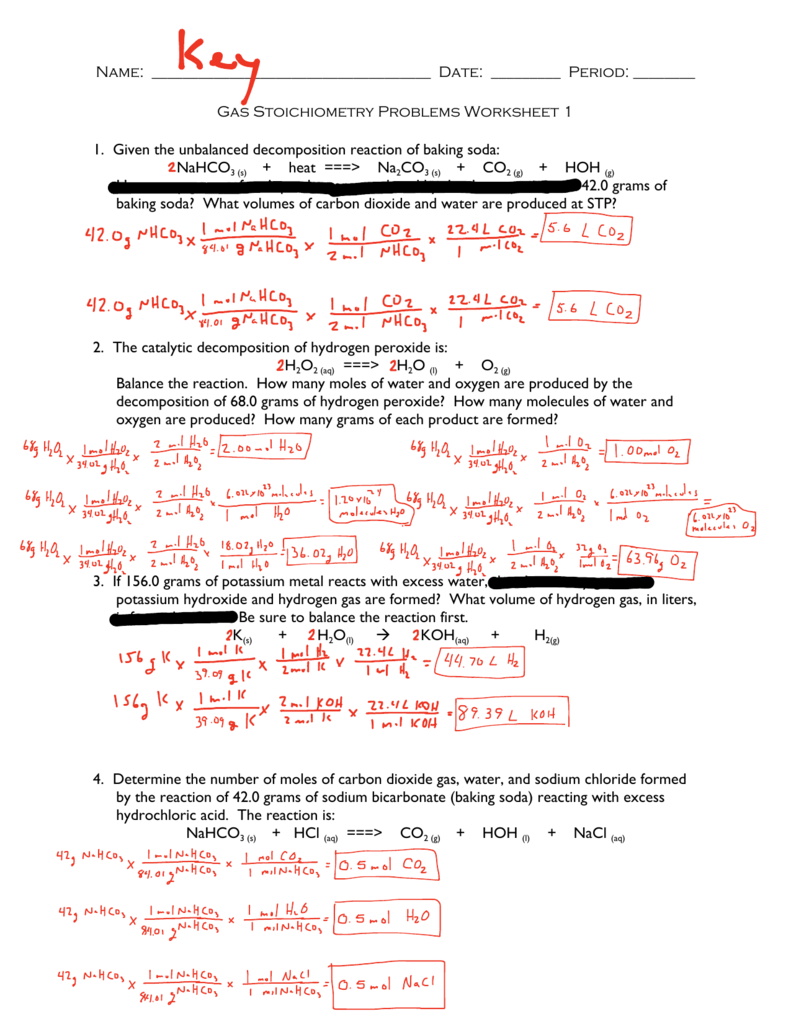
Unveiling the Secrets of Stoichiometry

Stoichiometry, an essential branch of chemistry, plays a pivotal role in understanding how substances interact. This article will guide you through the 5 steps to mastering this crucial aspect of chemistry with the help of our exclusive Worksheet Key. Whether you're a student grappling with these concepts for the first time or an enthusiast revisiting the basics, let's dive into simplifying stoichiometry.
Step 1: Understanding the Balanced Equation

The foundation of stoichiometry is the balanced chemical equation. This equation not only gives you the substances involved in a reaction but also the exact molar ratios of the reactants and products.
- Identify reactants and products
- Check for balance
- Understand stoichiometric coefficients
💡 Note: The key to stoichiometry lies in the balance. If an equation isn't balanced, you can't accurately predict quantities.
Step 2: Molar Mass and Molar Ratios

Once you have a balanced equation, you can calculate the molar mass of each substance involved. Molar mass is the mass of one mole of a substance and is used to convert between mass and moles.
- Find the atomic mass of each element in the equation
- Sum these to get the molar mass
- Use molar ratios to relate substances
Step 3: Limiting Reactants and Excess Reactants

In many reactions, one reactant will be entirely consumed before the others. This reactant is known as the limiting reactant, and it determines how much product can be formed.
| Substance | Moles Available | Moles Needed | Status |
|---|---|---|---|
| Reactant A | 5 | 4 | Excess |
| Reactant B | 3 | 3 | Limiting |

By comparing moles needed versus moles available, you can identify the limiting reactant.
Step 4: Moles to Mass Conversion

Now, let's convert moles into grams or vice versa. This step is crucial for practical applications, like determining how much of a substance can be produced in a chemical reaction.
- Use molar mass to convert moles to mass
- Or mass to moles using dimensional analysis
Step 5: Yield Calculations

Not all reactions proceed to completion. We calculate the yield, which can be either actual or theoretical.
- Theoretical Yield - based on stoichiometric calculations
- Actual Yield - what's obtained in the lab
- Percent Yield - the ratio of actual to theoretical
🧪 Note: The percent yield gives you an idea of the reaction's efficiency. If it's less than 100%, it indicates some loss or incomplete reaction.
In the journey to master stoichiometry, understanding the steps and their interrelations is key. Each step builds on the last, from interpreting the equation to calculating the yield. This 5-step guide, complete with our exclusive worksheet key, demystifies stoichiometry, making it an approachable and even enjoyable part of chemistry.
What is the difference between a limiting and an excess reactant?
+
The limiting reactant is the substance that’s completely consumed first in the reaction, limiting the amount of product that can be formed. An excess reactant has more moles than needed for the reaction to go to completion, leaving some unreacted.
Why is it important to balance a chemical equation?

+
Balancing an equation ensures that the Law of Conservation of Mass is upheld. It shows the correct molar ratios and amounts of reactants and products involved in the reaction, crucial for stoichiometric calculations.
How do you calculate percent yield?

+
To calculate percent yield, you use the formula: % Yield = (Actual Yield / Theoretical Yield) * 100
where Theoretical Yield is derived from stoichiometry, and Actual Yield is what’s physically obtained in the lab.