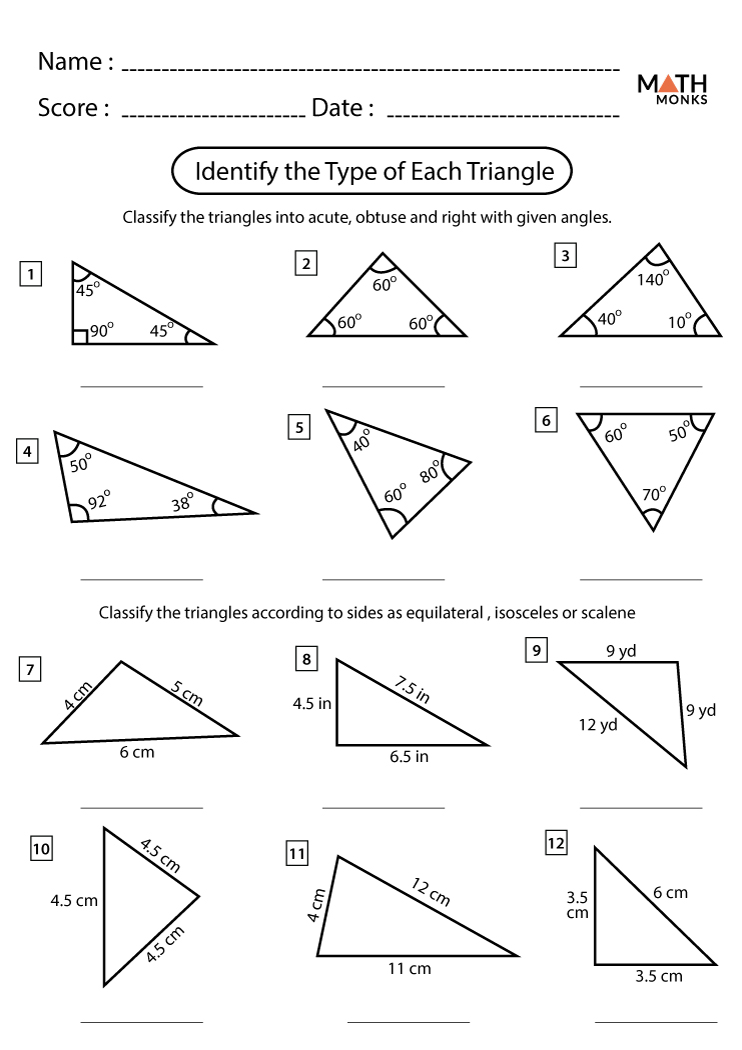
Stoichiometry Worksheet and Key: Master Chemical Calculations

Are you struggling to make sense of the numbers and formulas when it comes to chemistry? Stoichiometry, the calculation of reactants and products in chemical reactions, can be a daunting task. But with the right approach, you can become a pro at these calculations. This article will delve into stoichiometry through an interactive worksheet and key, providing practical examples, step-by-step solutions, and tips to enhance your understanding.
Understanding Stoichiometry

Stoichiometry involves using balanced chemical equations to calculate the quantities of reactants and products involved in a reaction. Here's what you need to know:
- Balanced Equations: Every stoichiometric calculation starts with a balanced chemical equation.
- Molar Ratios: The ratio derived from the coefficients in a balanced equation is used to relate the amounts of reactants to products.
- Molar Mass: Converting mass to moles or vice versa requires the molar mass of compounds.

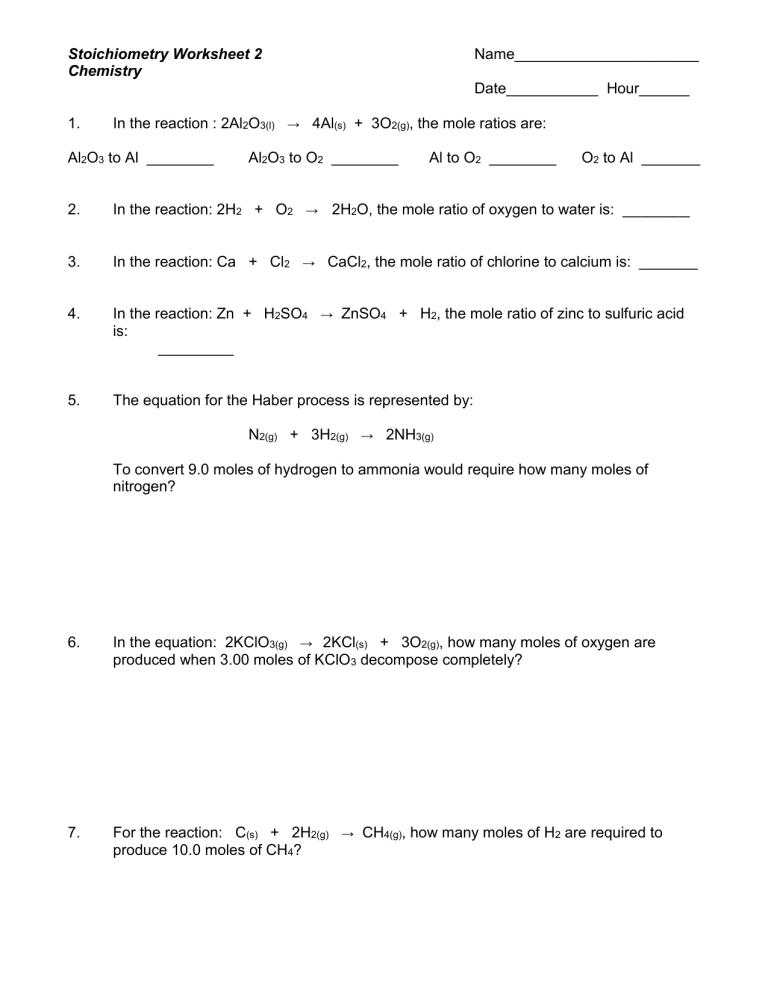
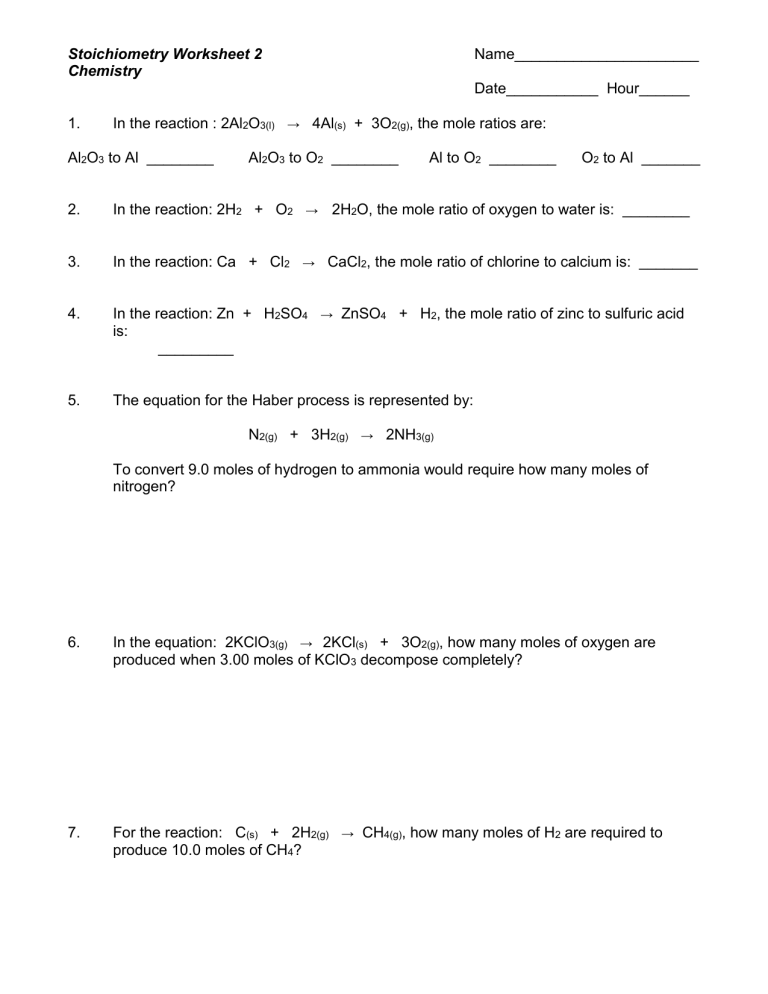
Stoichiometry Worksheet

Here is a simple stoichiometry worksheet to help you practice:
| Question | Solution |
|---|---|
|
If 12.0 grams of C6H12O6 (glucose) reacts completely with oxygen in the following reaction: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O What mass of CO2 will be produced? |
Step 1: Determine the molar mass of glucose and CO2. Step 2: Use the balanced equation to find the molar ratio between glucose and CO2. Step 3: Calculate moles of CO2 produced from the given mass of glucose. Step 4: Convert moles of CO2 to mass. |
Solution Steps:

Let's break down the steps for the above problem:
- Find Molar Masses:
- C6H12O6: 6 * 12.01 + 12 * 1.008 + 6 * 16 = 180.156 g/mol
- CO2: 44.01 g/mol
- Molar Ratio: From the equation, for every 1 mole of glucose, 6 moles of CO2 are produced.
- Moles of CO2: Mass of Glucose / Molar Mass of Glucose = 12.0 g / 180.156 g/mol = 0.0666 moles of glucose. Since the ratio is 1:6, 0.0666 moles of glucose produces 6 * 0.0666 = 0.3996 moles of CO2.
- Mass of CO2: Moles of CO2 * Molar Mass of CO2 = 0.3996 * 44.01 g/mol = 17.6 g CO2.
🔍 Note: Make sure all units are consistent when performing these calculations to avoid errors.
Additional Stoichiometry Problems
Below are a few more problems to practice:
Given the reaction:
2 Al + 3 Br2 → 2 AlBr3
Calculate the mass of aluminum required to produce 150 grams of AlBr3.
Consider the combustion of methane:
CH4 + 2 O2 → CO2 + 2 H2O
Determine the volume of CO2 at STP if 25 g of methane is burned.
In the synthesis of ammonia:
N2 + 3 H2 → 2 NH3
Find the mass of hydrogen needed to produce 10 kg of ammonia.
Summarizing the Learning

Stoichiometry is an integral part of chemistry that helps you understand how to predict chemical outcomes based on known reactant quantities. Here are the key takeaways:
- Balancing equations is crucial for accurate calculations.
- Using molar ratios helps in determining the quantities of reactants and products.
- Converting between mass and moles using molar mass is a common step.
By practicing with worksheets and reviewing keys, you can become proficient in stoichiometry, making chemical calculations less daunting and more understandable.
What are stoichiometric coefficients?

+
Stoichiometric coefficients are the numbers in front of substances in a balanced chemical equation, representing the moles of each substance involved in the reaction.
How do I use stoichiometry for a limiting reactant problem?

+
To solve a limiting reactant problem, calculate how much product each reactant would produce. The reactant producing the least amount of product is the limiting reactant.
What is the significance of Avogadro’s number in stoichiometry?

+
Avogadro’s number (6.022 x 10^23 particles per mole) is used to relate the amount of substance in moles to the number of particles, which is fundamental for stoichiometric conversions.
Can stoichiometry be applied to everyday life?

+
Yes, stoichiometry can be used in cooking, where recipes are essentially stoichiometric ratios, in manufacturing processes, and even in environmental analysis for pollutant control.
What common mistakes should I avoid in stoichiometry?

+
Common mistakes include: Not balancing equations, mixing up coefficients with subscripts, not converting units consistently, and ignoring the limiting reactant when calculating product yield.