Top 5 Tips for Solving Percent Yield Problems

Understanding and effectively solving percent yield problems can be a crucial skill, especially for students and professionals in chemistry, engineering, and related sciences. Percent yield calculations help in determining the efficiency of chemical reactions, guiding practical lab work, and optimizing industrial processes. Here are five comprehensive tips to master this essential topic:
1. Grasp the Basics of Percent Yield
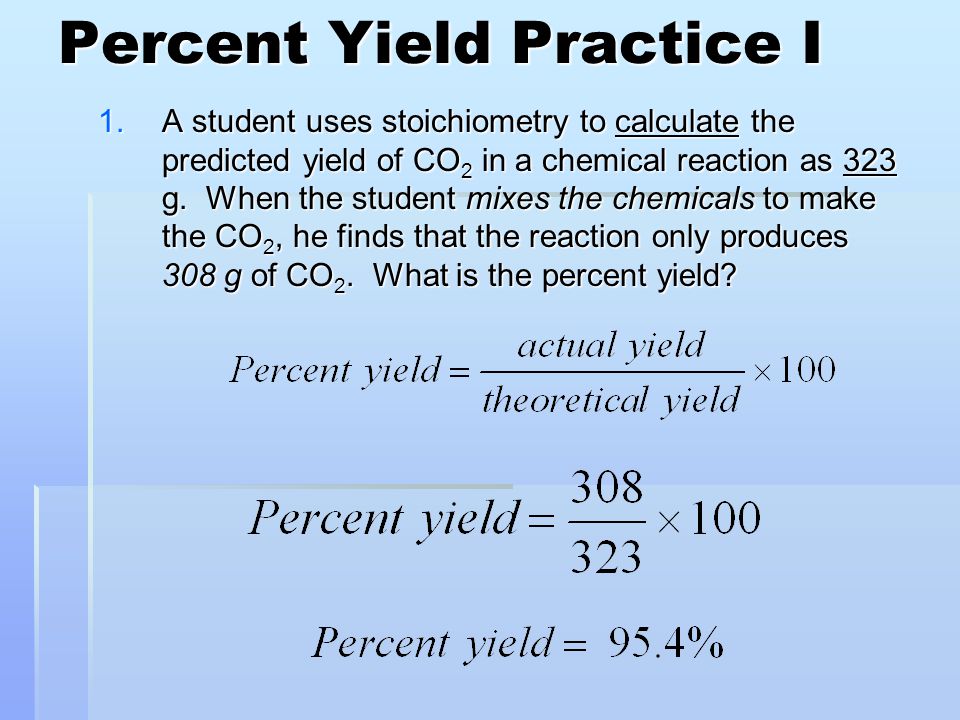
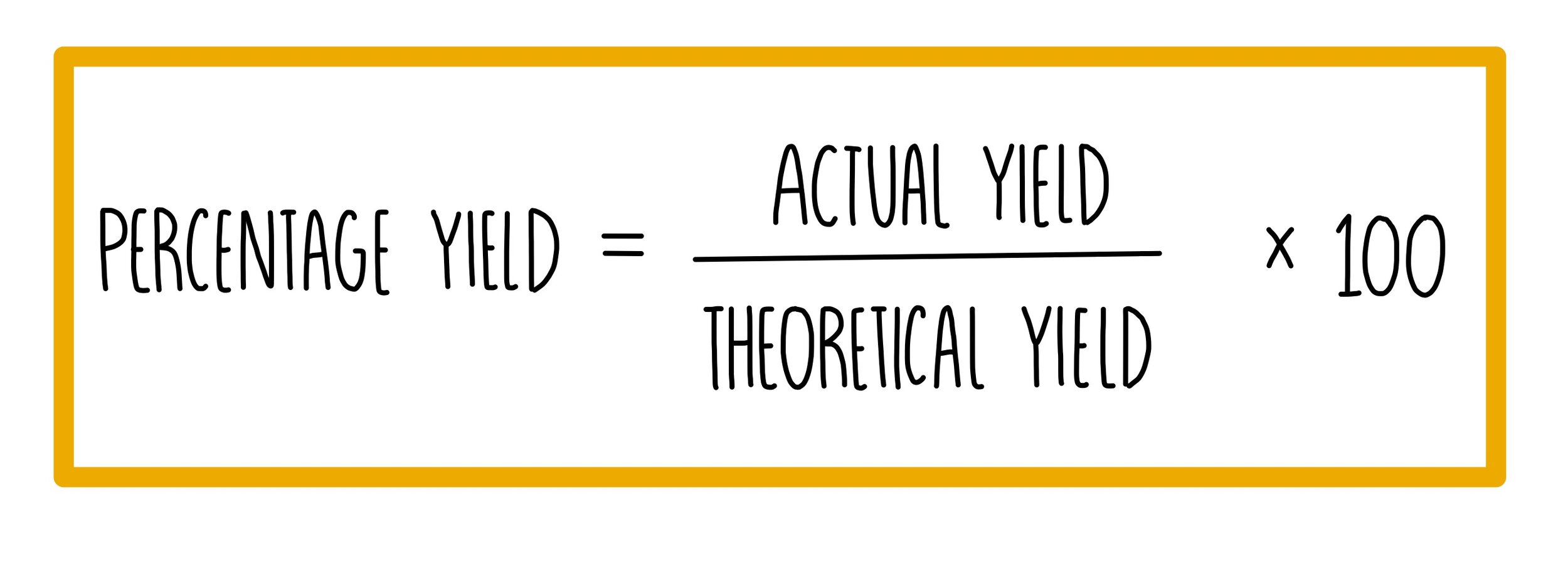
Before diving into complex problems, it’s vital to understand what percent yield represents. Percent yield is defined as the ratio of the actual yield of a reaction to its theoretical yield, multiplied by 100. This gives you a percentage that reflects how close you are to achieving the maximum possible yield of your chemical reaction.
- Actual Yield: The amount of product actually produced in a lab.
- Theoretical Yield: The amount of product that would be obtained if the reaction went to completion and there was no loss.
⚗️ Note: Percent yield is always less than or equal to 100% due to impurities, side reactions, or losses during transfer or filtration.
2. Accurate Determination of Theoretical Yield

The accuracy of your percent yield calculation heavily relies on the correct calculation of the theoretical yield. Here’s how to do it:
- Start by identifying the limiting reactant, the substance that determines how much product can be formed.
- Balance the chemical equation carefully, understanding the stoichiometric relationships between reactants and products.
- Calculate the moles of the limiting reactant available using its molar mass.
- Use the stoichiometry from the balanced equation to find how many moles of product can be made.
- Convert moles of product to grams using the product's molar mass.
3. Watch Out for Units and Conversions

Units can be one of the most common sources of errors in percent yield calculations. Here are some key points:
- Ensure all measurements are in the same units (grams, kilograms, moles, etc.).
- Use conversion factors if necessary, especially for complex units like cubic centimeters or liters to grams.
- Make sure the units for actual yield and theoretical yield are identical when calculating percent yield.
🚦 Note: Consistency in units is crucial. A mistake here can lead to significant errors in your calculations.
4. Consider Reaction Conditions and Efficiency
Real-world reactions don’t always proceed as expected due to various factors:
- Side reactions: Unwanted reactions that produce products other than the desired one.
- Heat and temperature: Incorrect temperature can slow down or stop the reaction, reducing yield.
- Impurities: Contaminants can react with your reactants or dilute the product.
- Equipment limitations: Incomplete mixing, surface area issues, or improper agitation can affect yield.
Understanding these variables helps in predicting and improving the efficiency of your reaction.
5. Practice and Real-World Application

Like any skill, proficiency in percent yield calculations comes with practice:
- Work through various example problems from textbooks, online resources, or even past lab reports.
- Reflect on your lab experiences, trying to correlate your percent yield with the conditions under which the reaction was performed.
- Discuss your results with peers or mentors to gain insights from different perspectives.
Moreover, apply these calculations in real-world scenarios:
- Manufacturing: Ensure optimal production yield by reducing waste and increasing efficiency.
- Pharmaceuticals: Maximize the yield of active ingredients to reduce costs and increase profitability.
- Environmental Science: Calculate the effectiveness of chemical treatments or processes.
By integrating these strategies, you not only improve your understanding of percent yield but also enhance your problem-solving skills in chemistry and related fields.
In the journey of mastering percent yield calculations, patience and persistent practice are your most valuable assets. Understanding the principles behind the calculation, ensuring accuracy in every step, and learning from real-world applications will empower you to tackle even the most challenging problems. Keep refining your technique, always staying mindful of units, reaction conditions, and the inherent limitations of laboratory work. This expertise will serve you well in academia and beyond, providing a solid foundation for optimizing chemical processes and exploring new scientific frontiers.
Why is my percent yield less than 100%?

+
A percent yield less than 100% is normal due to factors like side reactions, impurities, losses during transfer, incomplete reactions, and equipment limitations which prevent the reaction from reaching its theoretical maximum.
How can I improve my percent yield?

+
To enhance percent yield, you might consider: optimizing reaction conditions (temperature, pressure), ensuring reactants are pure, using catalysts or improving mixing, reducing side reactions, and minimizing loss during product isolation.
What does a 100% yield mean?

+
A 100% yield means that you have achieved the maximum possible amount of product from the reaction based on the stoichiometry of the reactants. However, this is rare due to the practical limitations mentioned earlier.