Master Stoichiometry with Worksheet 2: Easy Steps Explained

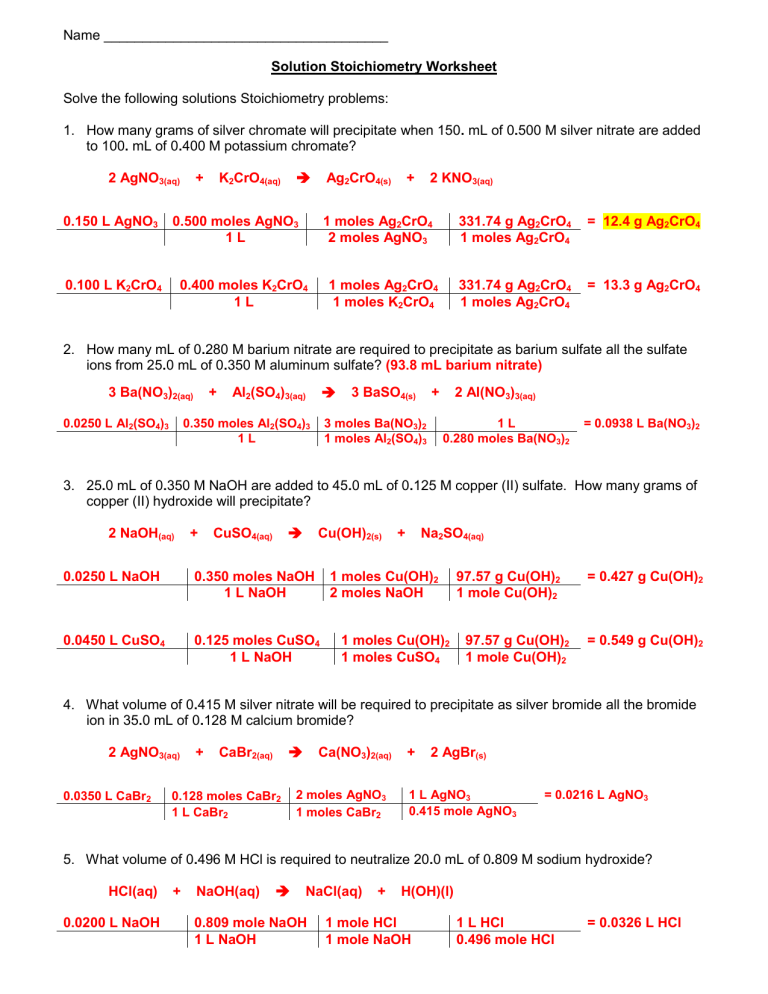
Stoichiometry is often a daunting topic in chemistry, with its intricate calculations and equations, but understanding it opens up the world of quantitative chemistry. This guide, with the help of Stoichiometry Worksheet 2, aims to demystify this crucial subject by breaking it down into digestible, easy-to-follow steps. Through this post, we will delve into how to solve stoichiometry problems efficiently, ensuring that you master this essential skill for your chemistry studies.
Understanding Stoichiometry: A Recap

Stoichiometry is the calculation of reactants and products in chemical reactions. It’s based on the law of conservation of mass and the principle that the number of moles of each element or compound involved in a reaction must be conserved. Here’s a quick recap:
- Balanced Chemical Equations: They ensure that the number of atoms of each element is the same on both the reactant and product side.
- Moles: The basic unit used to measure the amount of substance in stoichiometry.
- Mole Ratios: These are derived from the coefficients in balanced equations and are used to convert between reactants and products.
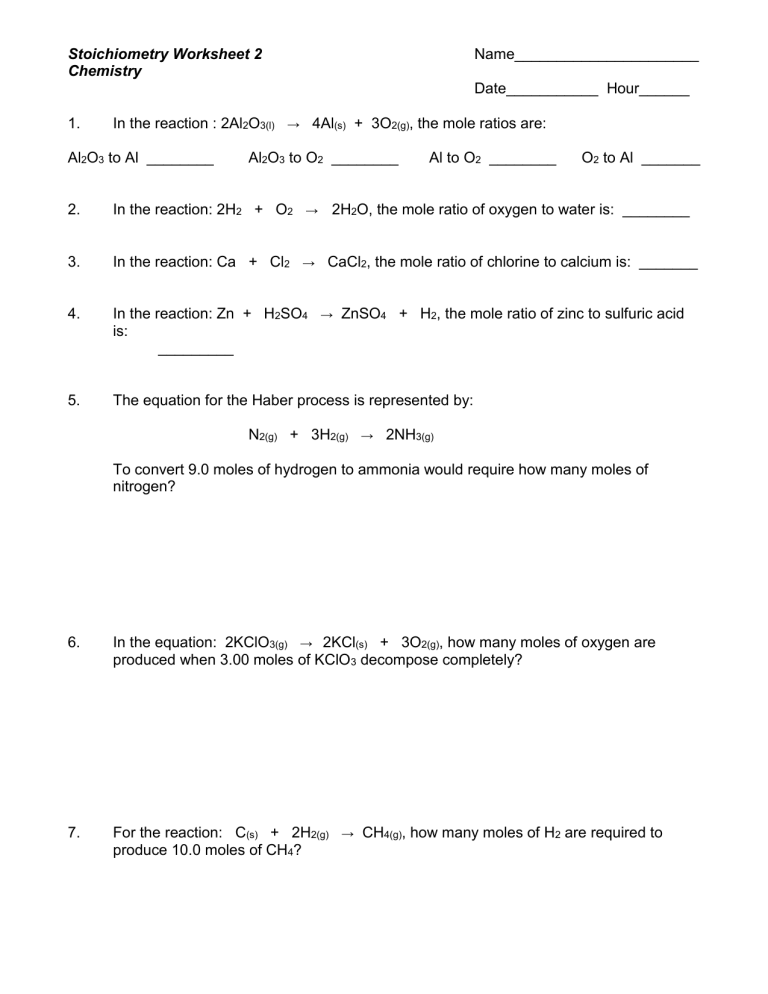
Mastering Stoichiometry Worksheet 2

Let’s dive into the practical aspects with our “Stoichiometry Worksheet 2.” This worksheet focuses on simple yet fundamental problems to build confidence in stoichiometry calculations.
Step 1: Analyzing the Balanced Equation

Every stoichiometry problem begins with a balanced chemical equation. Here’s what you need to do:
- Identify the reactants and products. Know what substances are reacting and what’s being formed.
- Check the coefficients. These numbers show the mole ratios in which substances react.

Step 2: Calculating Moles

The worksheet typically provides either:
- The amount of a reactant in grams.
- Or the volume of a gas at STP (Standard Temperature and Pressure).
Convert this amount into moles:
- For solids: Use molar mass.
- For gases: Use the Ideal Gas Law or the molar volume at STP (22.4 L/mol).
⚠️ Note: Ensure you have correctly identified the units and convert if necessary. For example, grams to moles using the molar mass or liters to moles using the molar volume at STP.
Step 3: Applying Mole Ratios

Using the coefficients from the balanced equation, you can calculate how many moles of another substance (reactant or product) will be involved or produced:
- Multiply the moles of the known substance by the appropriate mole ratio.
Here’s an example:
If the balanced equation is:
| Reactants | Moles | Products | Moles |
|---|---|---|---|
| 2H₂ | 1 | O₂ | 0.5 |
| —- | – | 2H₂O | 1 |
Step 4: Back to Grams or Moles

Once you’ve figured out the moles of the product or reactant you’re interested in, convert it back to grams using its molar mass or keep it in moles if the question asks for it:
- Convert moles to grams: Moles × Molar mass = Grams
Step 5: Verify Your Calculations

It’s beneficial to double-check your results to ensure:
- The stoichiometry makes sense within the context of the problem.
- Your units are consistent.
- All your calculations are accurate.
Application in Real-Life Scenarios
Stoichiometry isn’t just for textbooks. Here’s how it’s applied in various fields:
- Pharmaceuticals: Dosage calculations require precise stoichiometry to ensure safe and effective drug formulations.
- Environmental Science: Monitoring pollutants involves stoichiometric analysis to understand reaction pathways and outcomes.
- Industry: Chemical manufacturing processes rely on stoichiometry to optimize reactions, reduce waste, and ensure efficiency.

Conclusion

Mastering stoichiometry with “Stoichiometry Worksheet 2” takes patience and practice, but it’s rewarding. By following these steps and regularly revisiting your understanding through practice, you’ll gain confidence and expertise in this fundamental area of chemistry. Not only will you be able to tackle your homework and exams with ease, but you’ll also comprehend the real-world significance of these calculations.
What is the purpose of balancing a chemical equation in stoichiometry?

+
The purpose of balancing a chemical equation is to ensure that the law of conservation of mass is upheld, where the number of atoms of each element must be the same before and after the reaction.
How do you convert mass to moles in stoichiometry?

+
To convert mass to moles, divide the mass by the substance’s molar mass (mass/molar mass = moles).
Why is it important to understand the mole ratio in a reaction?

+
Understanding the mole ratio allows us to determine how much of each reactant is needed and how much product will be produced, ensuring the reaction can proceed as efficiently as possible.
Can stoichiometry be applied to real-life scenarios?

+
Yes, stoichiometry is crucial in fields like pharmaceuticals, environmental science, and manufacturing, where accurate calculations are essential for safety, efficiency, and product quality.
What should I do if I keep making errors in stoichiometry calculations?

+
Keep practicing and double-check your steps. Common errors include forgetting to balance the equation, incorrect unit conversion, or misinterpreting the question. Focus on understanding the underlying principles and slow down your calculation process.