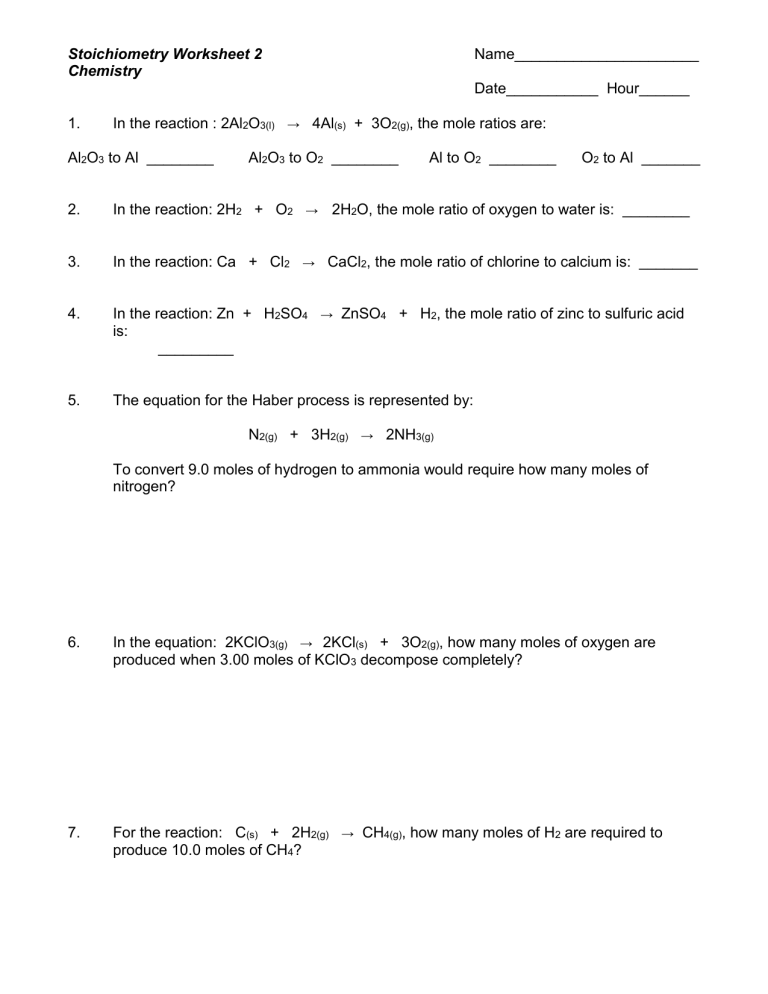
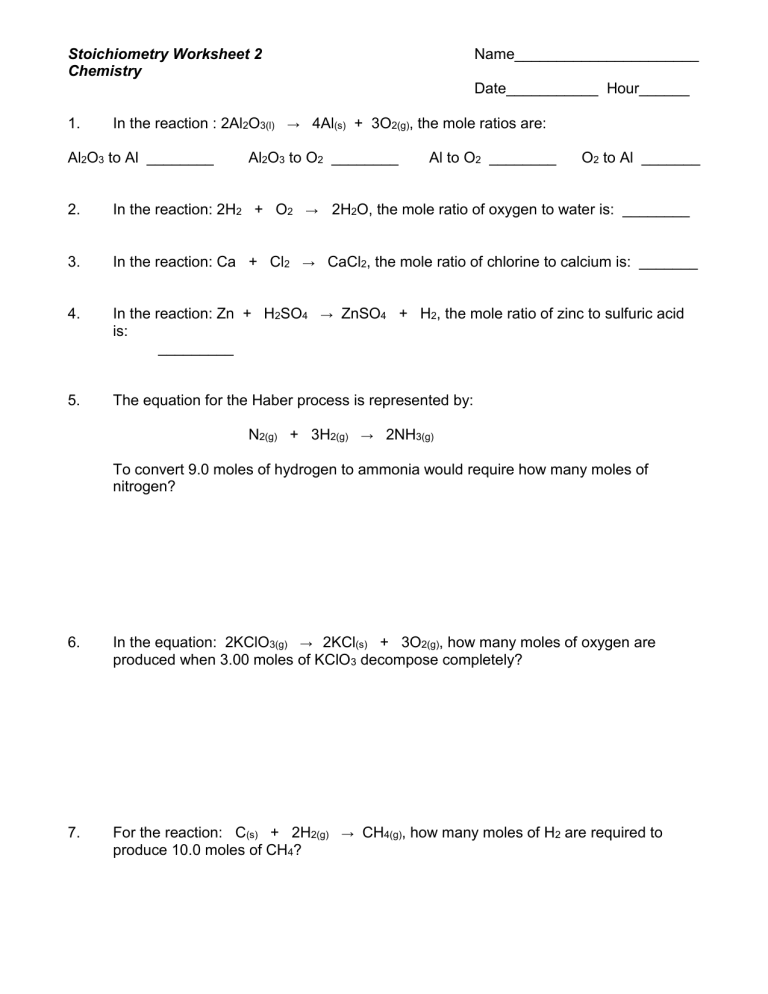
Stoichiometry Worksheet 1: Mole to Mole Calculations Key

In the realm of chemistry, mastering the art of stoichiometry is essential for students aspiring to delve deep into chemical reactions and synthesis. Today, we’ll walk through the fundamentals of mole to mole calculations, which form the backbone of stoichiometric analysis. These calculations are crucial for understanding how reactants interact at the molecular level, aiding in predicting the outcomes of chemical reactions accurately.
What is Mole to Mole Calculation?
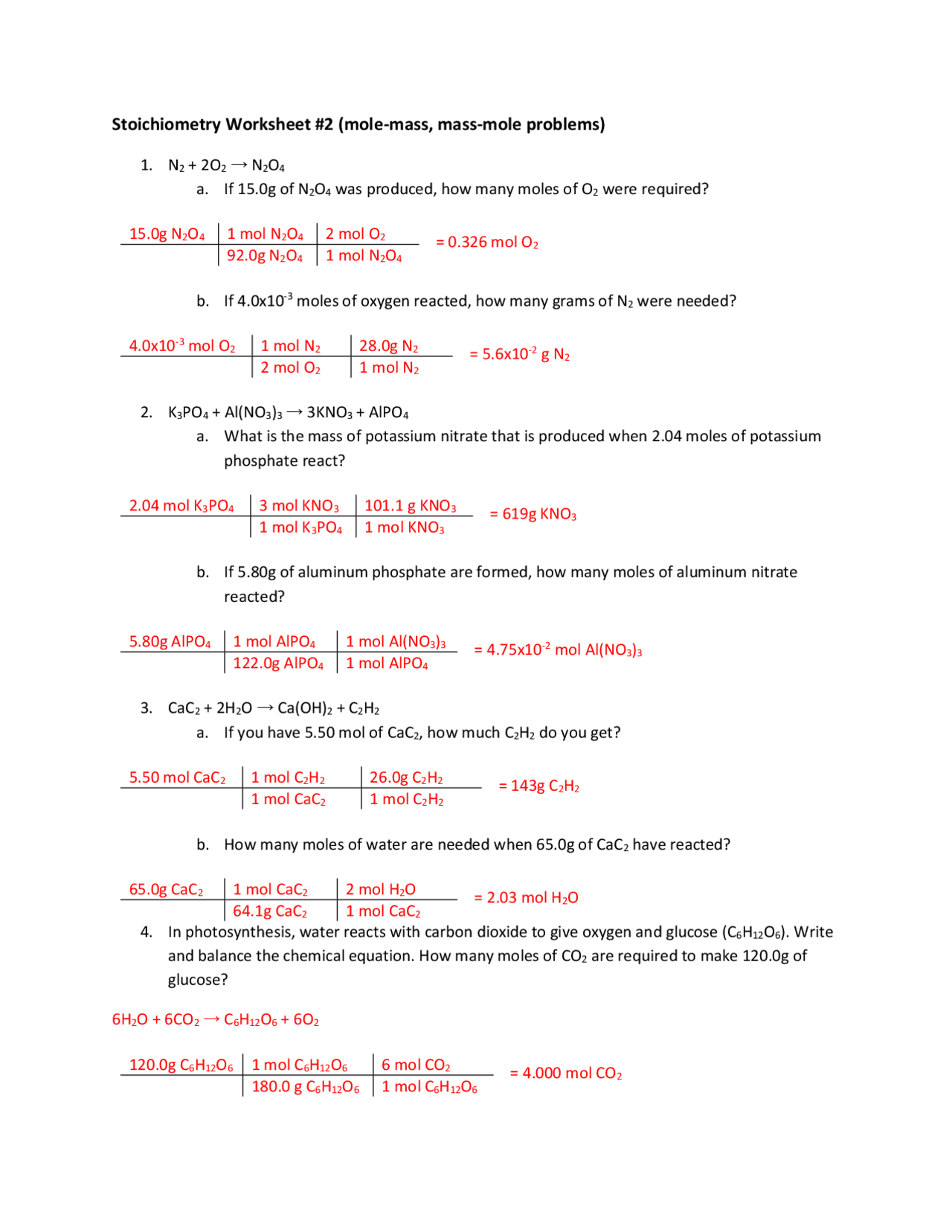

Before we dive into the calculations, let’s understand what a mole is. A mole (symbol: mol) is a unit of measurement that quantifies the amount of substance. It is defined as the number of atoms in 12 grams of carbon-12, which is 6.022 x 1023—Avogadro’s number.
⚗️ Note: Avogadro’s number is the key to converting between moles and molecules or atoms. It's like the atomic bridge between microscopic particles and measurable quantities.
How Does Mole to Mole Calculation Work?

Mole to mole calculations involve using balanced chemical equations to determine how many moles of one substance will react with or produce moles of another substance. Here’s a simple step-by-step guide:
- Start with a balanced chemical equation.
- Identify the given substance and the target substance.
- Use the coefficients from the balanced equation as the ratio to find the moles of the target substance.
| Step | Description |
|---|---|
| 1. Write the Balanced Equation | Ensure the chemical equation you use reflects the actual stoichiometric ratios of reactants and products. |
| 2. Identify Mole Ratios | From the balanced equation, determine the ratio of moles of the given substance to the moles of the desired substance. |
| 3. Calculate Moles | Use the identified ratio to calculate how many moles of the target substance will be produced or are needed. |

Let's illustrate this with an example:
Example Calculation
Given the reaction: 2H₂ + O₂ → 2H₂O
- Suppose we have 3 moles of hydrogen (H₂). How many moles of water (H₂O) can we produce?
- From the equation, the molar ratio of H₂ to H₂O is 2:2, which simplifies to 1:1.
- Therefore, 3 moles of H₂ will produce 3 moles of H₂O.
💡 Note: The key to solving these problems lies in understanding that the coefficients in a balanced chemical equation represent the molar ratios of the reactants and products involved.
Common Pitfalls in Mole to Mole Calculations

Here are some common errors students might encounter:
- Ignoring Balancing: Always work with a balanced chemical equation; unbalanced equations lead to incorrect stoichiometry.
- Confusing Molar Mass with Mole Ratio: Remember, the molar ratio comes from the coefficients, not the molecular weights or masses.
- Neglecting Limiting Reactants: In more complex problems, you must consider which reactant is the limiting factor and will be used up first.
Strategies to Master Mole to Mole Calculations
To enhance your proficiency in these calculations:
- Practice with different chemical equations.
- Understand the concept of limiting reagents.
- Make use of dimensional analysis to set up your calculations correctly.
🔍 Note: Always double-check your balanced equations to ensure they correctly represent the reaction before proceeding with mole calculations.
By this point, you should have a firm grasp of how to approach and solve mole to mole calculations. These fundamental principles are not only essential for understanding stoichiometry but are also vital for lab work where predicting the yield of reactions is crucial. Chemistry is a precise science where accuracy in calculations can mean the difference between success and failure in experiments or industrial processes.
Summarizing Our Exploration

In summary, mole to mole calculations are a cornerstone of quantitative chemistry, allowing us to predict outcomes, assess reaction efficiencies, and plan for synthesis with precision. By mastering these calculations, students and chemists alike can better understand the intricate dance of atoms and molecules during reactions, leading to better control over chemical processes.
Why are balanced chemical equations important in stoichiometry?

+
Balanced chemical equations provide the exact ratios of reactants and products, which are essential for accurate mole to mole calculations. Without a balanced equation, the stoichiometry would be incorrect, leading to skewed predictions or results.
What is the difference between mole ratio and molar mass?
+
The mole ratio is the numerical relationship between coefficients in a balanced chemical equation, while molar mass is the mass of one mole of a substance, which is typically expressed in grams per mole (g/mol).
How do you find the limiting reagent in a reaction?

+
To find the limiting reagent, you calculate how much product each reactant would produce if used in excess. The reactant that produces the least amount of product is the limiting reagent, as it limits the reaction’s extent.