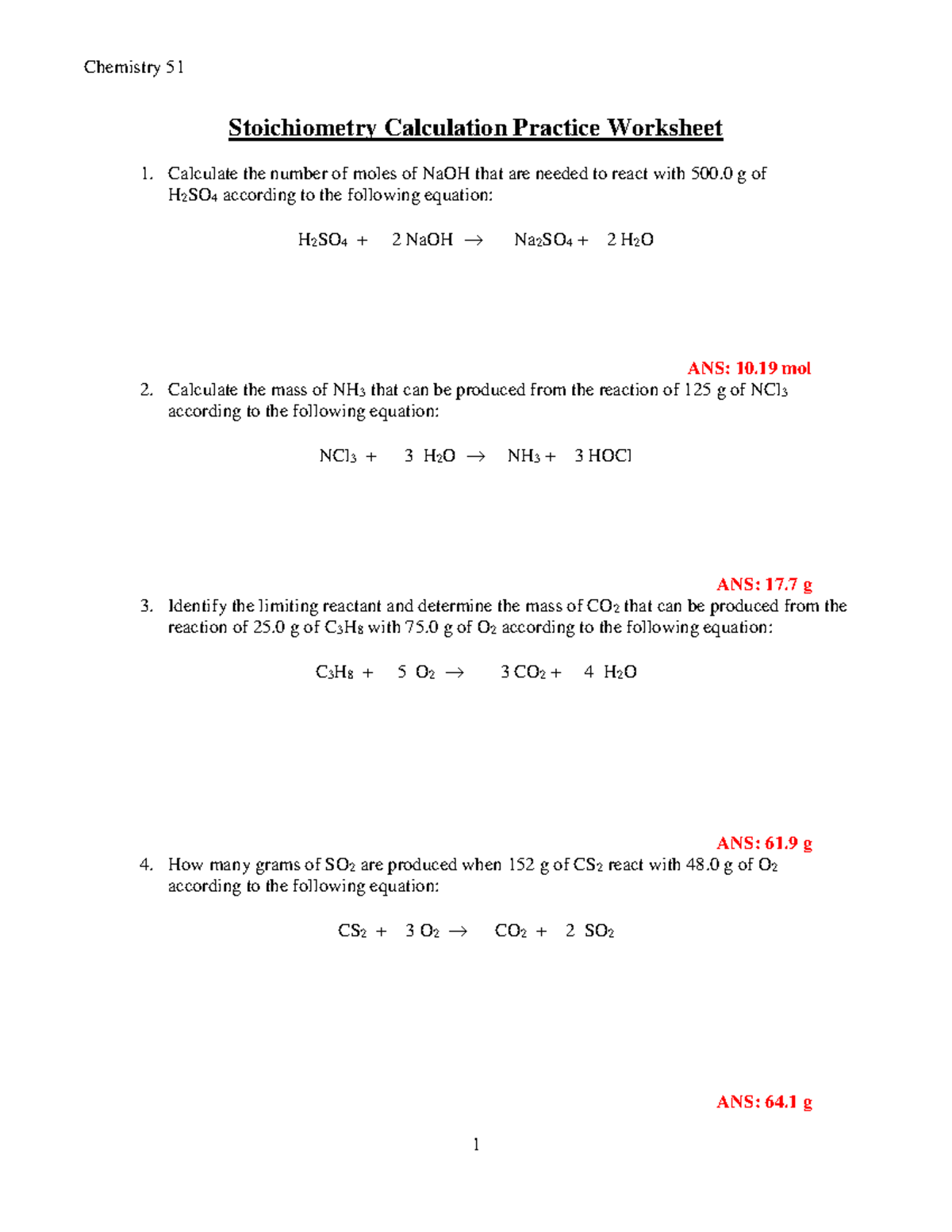
Solve Stoichiometry Problems Easily with This Chem Worksheet Guide

In the realm of chemistry, stoichiometry stands as a fundamental pillar, crucial for understanding chemical reactions and their quantitative aspects. Whether you're a student grappling with the basics or an enthusiast looking to master chemistry, this guide offers a streamlined approach to solving stoichiometry problems using a worksheet framework.
Understanding Stoichiometry: The Basics

Stoichiometry involves the calculation of reactants and products in chemical reactions. Here’s what you need to know:
- Balanced Equations: The foundation of stoichiometry is a balanced chemical equation where the number of atoms for each element remains constant before and after the reaction.
- Mole Concept: It’s essential to understand moles because stoichiometry relies on the molar ratios between reactants and products.
- Limiting and Excess Reactants: In most real-world scenarios, one reactant will limit the reaction, while others are in excess.
Basic Steps for Solving Stoichiometry Problems

Let’s break down the process of tackling stoichiometry problems using a worksheet guide:
Step 1: Write and Balance the Chemical Equation

First, you must have a balanced equation. Here’s how:
- Identify the reactants and products.
- Balance the equation by adjusting the coefficients so that the number of atoms of each element on both sides is equal.
Step 2: Identify Knowns and Unknowns

What information do you have, and what do you need to find? This includes:
- Amounts of reactants (in grams or moles)
- The desired amount of product or another reactant
Step 3: Convert to Moles
Convert any mass quantities to moles using the molar mass of the substances. Use the following formula:
Moles = (Given Mass) / (Molar Mass)
Step 4: Apply Mole Ratios

Use the coefficients from the balanced equation to set up ratios. For example:
- 2H2 + O2 → 2H2O gives a ratio of 2:1:2 for H2:O2:H2O.
Step 5: Calculate Masses or Volumes

If needed, convert back from moles to mass using the molar mass or to volume for gases at STP:
- Mass = Moles × Molar Mass
- Volume = Moles × Molar Volume (22.4 L at STP)
Step 6: Limiting Reactant and Excess Determination

Determine which reactant will run out first:
- Calculate how much product can be formed by each reactant.
- The reactant producing the least amount of product is the limiting reactant.
Step 7: Analyze the Results

After calculating, ensure your results make sense with the given information. Double-check:
- Units match your requirements.
- The stoichiometry is correctly applied.
- Conversions from mass to moles and back are accurate.
💡 Note: Practice with various problems to get a feel for common pitfalls like unit conversions and not balancing equations properly.
Common Pitfalls and How to Avoid Them

Here are some frequent mistakes in stoichiometry:
- Not Balancing the Equation: This leads to incorrect mole ratios. Always check your equation.
- Using Incorrect Molar Masses: Double-check the periodic table or provided information for the correct masses.
- Mistakes in Mole Conversions: Moles are the key; ensure you convert mass to moles accurately.
- Ignoring Limiting Reactants: Understand which reactant dictates the reaction’s limits.
Visualizing Stoichiometry: A Practical Table

Here’s a simple table to help visualize the stoichiometric relationship in a reaction:
| Compound | Formula | Molar Mass (g/mol) | Moles Given | Moles Produced |
|---|---|---|---|---|
| Hydrogen | H2 | 2.016 | 1 | 0 |
| Oxygen | O2 | 32.00 | 0.5 | 0 |
| Water | H2O | 18.02 | 0 | 1 |

Advanced Stoichiometry Techniques

Beyond the basics, here are techniques for more complex scenarios:
- Solutions: When dealing with solutions, consider molarity and dilution.
- Gas Laws: Incorporate the ideal gas law for reactions involving gases.
- Percent Yield: Calculate how close your actual yield is to the theoretical yield.
By mastering these steps and understanding the potential pitfalls, you can tackle stoichiometry problems with confidence. This guide provides a structured approach to problem-solving, ensuring that you cover all necessary bases in your calculations.
Why is balancing a chemical equation important in stoichiometry?

+
Balancing a chemical equation ensures that the law of conservation of mass is observed, providing accurate mole ratios for calculations.
What if I have masses instead of moles in a problem?

+
Convert the masses to moles using the molar mass of each substance before applying mole ratios from the balanced equation.
How do I determine the limiting reactant?

+
Compare the amounts of product that each reactant would produce if completely reacted. The reactant yielding the least amount of product is the limiting reactant.
Can stoichiometry apply to everyday scenarios?
+Yes, stoichiometry is used in cooking, industrial processes, environmental engineering, and even in pharmacology for drug formulation.