5 Essential Answers to States of Matter and Phase Changes
The states of matter and the transitions between them are fundamental concepts in physical science. These concepts explain not only how substances behave at different temperatures and pressures but also how these behaviors govern much of the physical world around us. Understanding states of matter and phase changes is crucial for students, educators, and anyone interested in the sciences. This blog post will delve into five essential questions about states of matter, providing a comprehensive understanding of what they are, how they change, and their implications.
1. What Are the States of Matter?

The primary states of matter are:
- Solid: Particles are closely packed, with a fixed shape and volume.
- Liquid: Particles are close together but can move past each other, maintaining a constant volume but taking the shape of the container.
- Gas: Particles are far apart and in rapid motion, filling the entire volume of their container with no fixed shape.
- Plasma: A hot, ionized gas, often seen in stars or neon signs, where particles are even more energetic and partially ionized.
- Bose-Einstein Condensate: A state of matter that occurs at near absolute zero where particles occupy the same quantum state.
2. How Do Phase Changes Occur?

Phase changes are transitions between the different states of matter and are governed by:
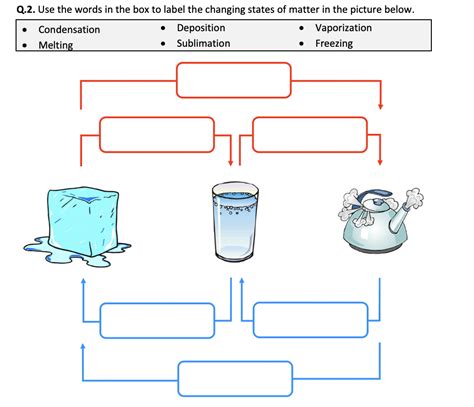
- Melting: The transition from solid to liquid. It occurs when enough heat is supplied to overcome the forces holding the solid structure.
- Freezing: The transition from liquid to solid. When the temperature is lowered enough for the particles to form a crystalline lattice.
- Vaporization: The change from liquid to gas, which can be either evaporation at any temperature or boiling at a specific boiling point.
- Condensation: The gas to liquid phase change when particles lose enough energy to come together.
- Sublimation: The solid directly turning into gas, bypassing the liquid phase.
- Deposition: The reverse of sublimation, where gas directly becomes a solid.
🌡️ Note: Phase changes require or release latent heat, which is the energy used to change the state without changing the temperature of the substance.
3. What Are the Conditions Required for Phase Changes?

The conditions necessary for phase changes include:
- Temperature: The average kinetic energy of the particles; phase changes often happen at specific temperatures known as melting points or boiling points.
- Pressure: External pressure can influence phase transitions; for instance, water can boil at lower temperatures at higher elevations due to lower pressure.
- Energy Input/Output: Adding energy (usually as heat) can promote transitions to higher-energy states (liquid to gas), while removing energy can lead to transitions to lower-energy states (gas to liquid).
4. How Do Temperature and Pressure Influence the States of Matter?

The interplay between temperature and pressure can be visualized through:
- Phase Diagrams: These diagrams show the various states of matter as a function of temperature and pressure. Here’s a basic layout:
| Temperature | P1 | P2 | P3 |
|---|---|---|---|
| T1 | Solid | Liquid | Gas |
| T2 | Solid | Liquid | Gas |
| T3 | Solid | Gas | Gas |

In the table, P1, P2, P3 represent different pressures, and T1, T2, T3 represent different temperatures. Each state (solid, liquid, gas) is shown at different conditions, with the lines separating the areas representing phase transitions.
5. Why Is Understanding Phase Changes Important?

Knowledge of phase changes and states of matter has wide-reaching applications:
- Meteorology: Understanding how water vapor condenses to form clouds or how ice can sublimate into fog.
- Chemistry: It’s crucial for processes like distillation or the behavior of gases in chemical reactions.
- Engineering: Designing systems that involve heat transfer, refrigeration, or materials science.
- Food Science: Phase changes affect food texture, cooking methods, and preservation techniques.
- Environment: Knowledge about phase changes helps in understanding climate patterns, greenhouse gas behavior, and environmental impacts.
Understanding states of matter and phase changes provides us with the ability to predict and manipulate the physical behavior of substances. This knowledge has led to advancements in technology, health, safety, and our understanding of the universe. By exploring these phenomena, we not only enrich our scientific knowledge but also pave the way for practical applications in everyday life. From the simple act of boiling water to the complex dynamics of weather systems, phase changes are integral to our environment and daily experiences.
What is the difference between condensation and evaporation?

+
Condensation is the process where a gas or vapor changes into a liquid, which requires the release of energy. Conversely, evaporation is when a liquid changes into gas, absorbing energy from the environment in the process. Both are phase changes but occur in opposite directions.
Can solids directly turn into gases?

+
Yes, this process is known as sublimation, where a substance transitions from a solid to a gas state without going through the liquid phase. An everyday example is dry ice (solid carbon dioxide) sublimating into carbon dioxide gas.
Why does water expand when it freezes?

+
When water freezes, the hydrogen bonds between the molecules form a crystalline structure, which has a lower density than liquid water. This expansion upon freezing is unusual among liquids, making ice less dense, allowing it to float on water.