5 Essential Tips for Mastering Specific Heat Worksheets
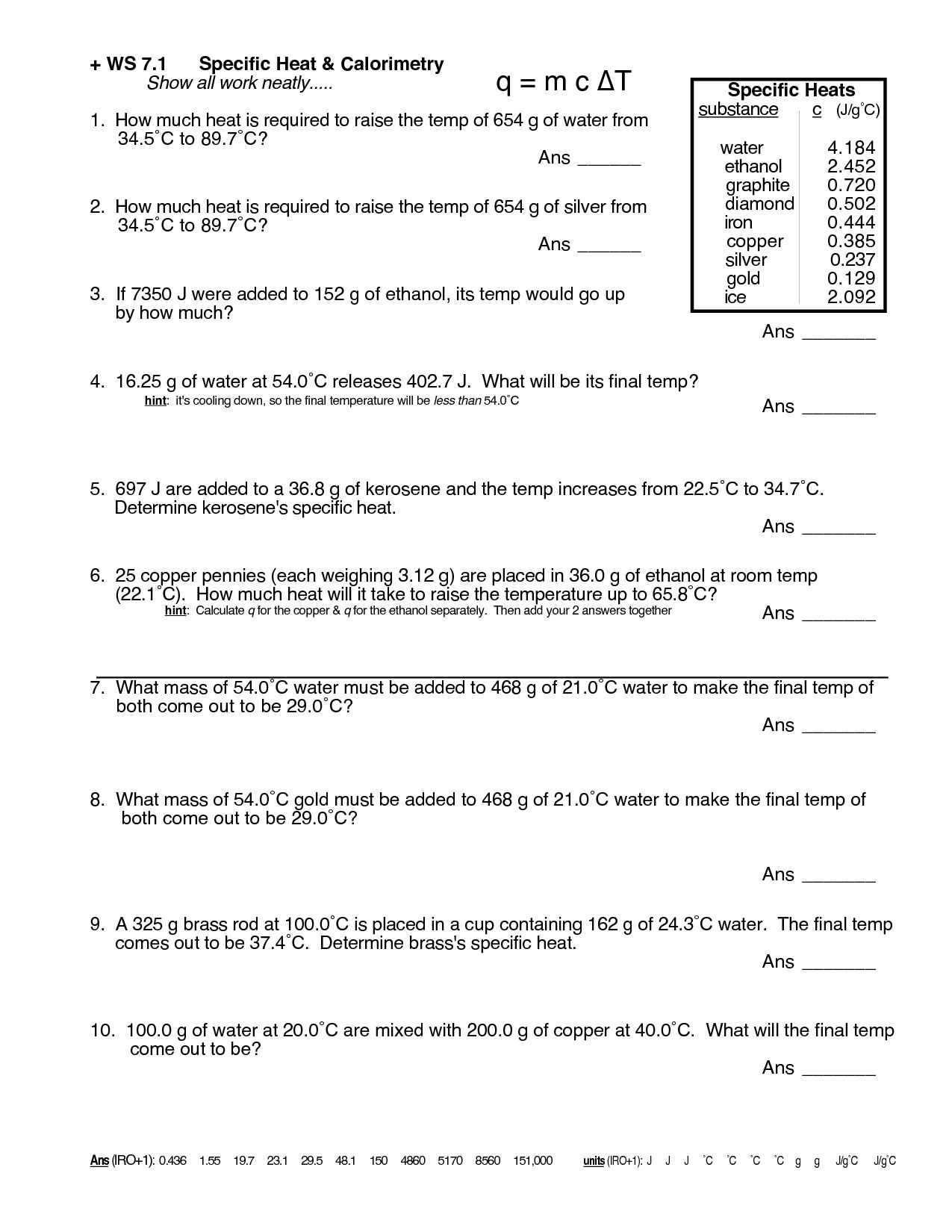
Understanding the Concept of Specific Heat

Specific heat, or specific heat capacity, is a crucial concept in thermodynamics that measures the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). This property is pivotal in various scientific and engineering applications, particularly when calculating the energy needed for heating or cooling substances. In this blog post, we will delve into essential tips that can help you master specific heat worksheets and problems effectively.

1. Grasp the Fundamentals

Understanding the basics is the foundation of mastering any subject:
- Definition: Specific heat capacity (c) is defined as c = Q / (m * ΔT), where Q is the heat energy absorbed or released, m is the mass of the substance, and ΔT is the change in temperature.
- Units: The common unit for specific heat capacity is joules per gram-degree Celsius (J/g°C).
- Molar Heat Capacity: If you're dealing with moles rather than mass, then you're dealing with molar heat capacity, which uses joules per mole-degree Celsius (J/mol°C).
2. Use Conversion Factors Efficiently

Conversion of units is often a source of error in thermodynamics problems:
- Remember to convert units if necessary. For instance, specific heat values might be listed in J/kg°C, but your problem could require grams.
- Conversion Example: To convert specific heat from J/kg°C to J/g°C, divide by 1000 (since 1 kg = 1000 g).
3. Apply the Heat Equation Correctly

The heat equation, Q = mcΔT, is your key to solving many specific heat problems:
- When given heat, mass, and the change in temperature, you can find specific heat by rearranging the equation: c = Q / (m * ΔT).
- If asked to calculate the final temperature, use the equation: ΔT = Q / (mc).
- Make sure to keep units consistent to avoid calculation mistakes.
⚗️ Note: Always check the units in your equations to ensure they are consistent. If they are not, convert them to maintain homogeneity in your calculations.
4. Understand Heat Transfer Mechanisms

Heat transfer involves three primary mechanisms: conduction, convection, and radiation:
- Conduction: Transfer of heat through direct contact or through a medium without significant movement of matter.
- Convection: Occurs in fluids where the transfer of heat happens due to the movement of matter within the fluid.
- Radiation: Emission of heat via electromagnetic waves.
In specific heat problems, understanding these mechanisms can help you to predict the behavior of substances under different conditions.

5. Master Multi-Step Problem Solving

Many problems in thermodynamics are multi-step processes:
- Identify each step involved in the problem (like heating, cooling, phase changes).
- Understand the energy transfer at each stage and apply the appropriate heat equations.
- Pay attention to any phase transitions, where specific heat capacity might change.
🔎 Note: When dealing with phase transitions, remember that the energy is used to change the phase, not to increase the temperature. This means you don't use the specific heat capacity for this part of the calculation.
Wrapping Up

Mastering specific heat worksheets requires a combination of understanding the fundamental concepts, applying equations correctly, and maintaining meticulous attention to detail. By following these tips, you'll enhance your problem-solving skills and gain confidence in handling thermodynamics problems. Practice using real-world scenarios, keep a reference of common specific heat values handy, and always double-check your calculations for accuracy. Remember, thermodynamics is not just about numbers; it's about understanding how energy flows and transforms, which is key to many natural and engineered systems.
What is the difference between specific heat capacity and heat capacity?

+
Heat capacity refers to the amount of heat needed to raise the temperature of a specific object by one degree. Specific heat capacity, however, is the heat capacity per unit mass, making it an intensive property of the material itself.
Can specific heat change with temperature?

+
Yes, for many substances, specific heat capacity can vary with temperature, especially when undergoing phase transitions. However, for most common conditions and in many calculations, it is treated as constant.
How do I calculate the energy required to heat water?

+
To calculate the energy required to heat water, you can use the equation Q = mcΔT, where Q is the energy in joules, m is the mass of water in grams, c is the specific heat of water (approximately 4.18 J/g°C), and ΔT is the change in temperature.
Why is specific heat important?

+
Specific heat is crucial for energy calculations in engineering, design of heating and cooling systems, understanding weather patterns, and many other fields where heat transfer is involved.
Can I use specific heat for chemical reactions?

+
Yes, specific heat can be used to calculate the heat absorbed or released during reactions, particularly when considering the enthalpy change of the system.