5 Easy Steps to Solve Specific Heat Problems

Mastering specific heat problems is crucial for anyone studying thermodynamics or working in related fields. Specific heat is a property of materials that tells us how much heat energy is needed to raise the temperature of a unit mass of a substance by one degree Celsius (or Kelvin). This concept becomes indispensable when designing systems, from cooling engines to creating thermal insulation materials. In this article, we will explore the 5 easy steps to tackle specific heat problems with confidence and precision.
Step 1: Understand the Specific Heat Formula

The first step in solving specific heat problems is to familiarize yourself with the specific heat formula:
Q = mc∆T
- Q: The amount of heat energy absorbed or released (measured in joules, J).
- m: The mass of the substance (measured in grams, g, or kilograms, kg).
- c: The specific heat capacity of the material (measured in J/g°C or J/kg°C).
- ∆T: The change in temperature (measured in °C or K).
Understanding this formula is essential as it forms the backbone of all calculations involving heat energy transfer.
Step 2: Identify Given Variables

Before you can solve any specific heat problem, you need to identify the known quantities:
- What is the mass of the substance?
- What is the initial and final temperature?
- What is the specific heat capacity of the material?
- How much heat energy is involved?
Here's an example of how to organize your data:
| Variable | Description | Value |
|---|---|---|
| m | Mass | 500 grams |
| c | Specific Heat Capacity | 4.18 J/g°C |
| ∆T | Change in Temperature | 20°C |

By identifying these variables, you can start to formulate your calculation.
🔍 Note: Be consistent with units; if they differ, convert them to a common standard before proceeding.
Step 3: Plugging the Values into the Formula
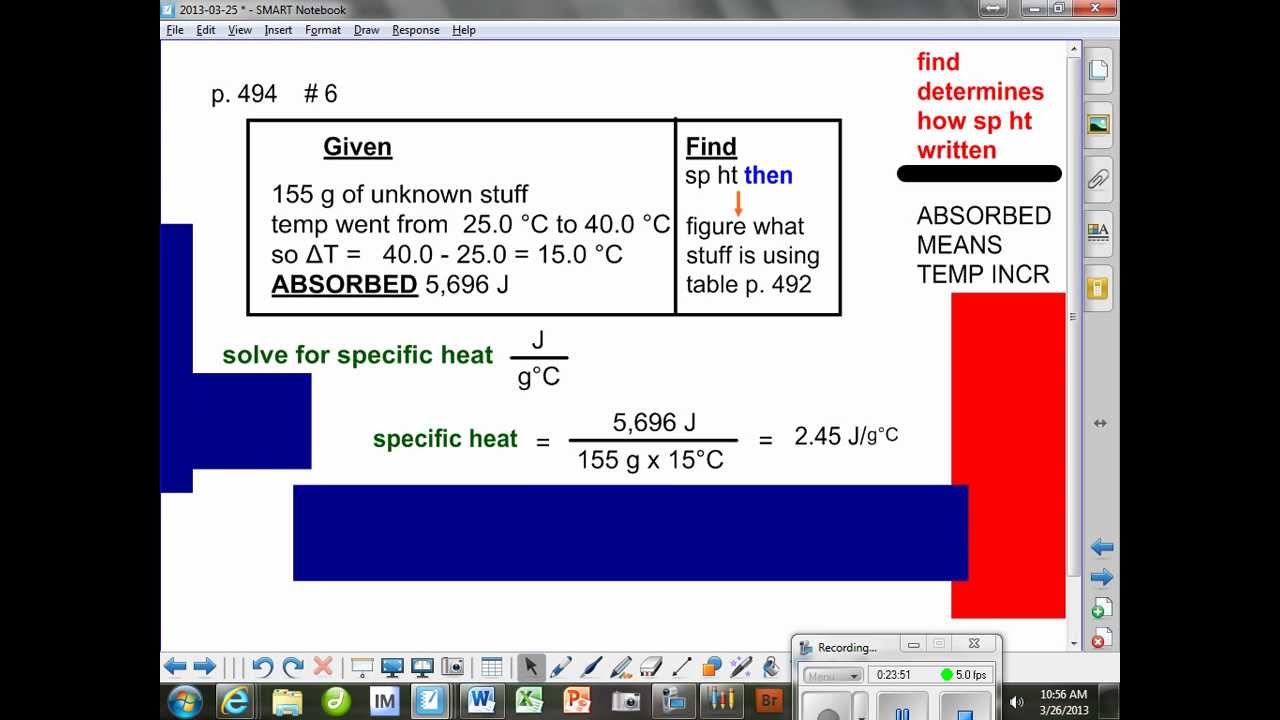
With the variables identified, plug them into the specific heat formula:
Q = mc∆T = (500 g) * (4.18 J/g°C) * (20°C)
This will give us the amount of heat energy in joules:
Q = 41,800 J
Remember to pay attention to the signs: positive Q indicates heat absorption, while negative indicates heat release.
Step 4: Calculate the Unknown Variable

Not all specific heat problems provide all four variables. Here's how to solve for an unknown:
- If Q is unknown:
- Q = mc∆T
- If m is unknown:
- m = Q / (c * ∆T)
- If ∆T is unknown:
- ∆T = Q / (m * c)
- If c is unknown:
- c = Q / (m * ∆T)
Example: If you know the mass, heat, and temperature change, but the specific heat capacity is unknown:
c = 41,800 J / ((500 g) * (20°C)) = 4.18 J/g°C
🔍 Note: Ensure your units are correct; mistakes in unit conversion can lead to incorrect results.
Step 5: Verify Your Results

After solving the problem, verification of your results is key:
- Check your calculations for arithmetic errors.
- Compare your result with typical values for the specific heat capacity of common substances.
- Does your answer make sense in the context of the problem?
- Check for any unit conversion issues that might have occurred.
By reviewing your work, you can ensure the reliability of your solution.
Understanding and solving specific heat problems opens up a world of possibilities in various scientific and engineering disciplines. With these 5 easy steps, you can confidently tackle any specific heat problem, ensuring accurate and insightful calculations. These steps not only help you to solve individual problems but also deepen your understanding of heat transfer, material properties, and energy conservation.
How do I convert units for specific heat capacity?

+
Converting units for specific heat capacity involves understanding that 1 J/g°C = 1 cal/g°C / 4.1868. To convert from J/g°C to J/kg°C, multiply by 1000 (since 1000 g = 1 kg).
Can I use specific heat to determine the energy required to heat a mixture of substances?

+
Yes, you can. First, calculate the heat required for each component individually using their specific heat capacities. Sum these values to get the total energy required for the mixture.
Why do some substances have high specific heat capacities?
+
Substances with high specific heat capacities, like water, have more complex molecular structures that can absorb and store more heat energy before their temperature increases significantly.
Is specific heat the same at all temperatures?

+
No, specific heat can vary with temperature, especially in solids and gases. However, for many substances, specific heat is relatively constant over a range of temperatures.
How does phase change affect specific heat problems?

+
During phase changes, like melting or boiling, the specific heat equation does not apply directly since the energy is used to change the state of the substance rather than changing its temperature.