5 Ways to Master Specific Heat Calculations

Mastering specific heat calculations can elevate your understanding of thermodynamics, a cornerstone of physical sciences. Whether you're a student grappling with fundamental principles or a professional requiring precise calculations for practical applications, this guide offers a comprehensive pathway to conquer this crucial concept. We'll navigate through the steps necessary to perform accurate heat calculations, enhancing your ability to predict energy transfers and their effects on matter.
Understanding Specific Heat

Specific heat, also known as specific heat capacity, is the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius or Kelvin. It’s a property unique to each material, often symbolized by (c) or (s), and is expressed in units like Joules per gram per Celsius degree (J/g·°C) or Joules per kilogram per Kelvin (J/kg·K).
Steps for Calculating Specific Heat

- Identify Known Variables: Determine what you know. This usually includes:
- The mass ((m)) of the substance in grams or kilograms.
- The temperature change ((ΔT)) in degrees Celsius or Kelvin.
- Either the heat added ((q)) or the specific heat capacity ((c)) of the substance.
- Use the Specific Heat Formula:
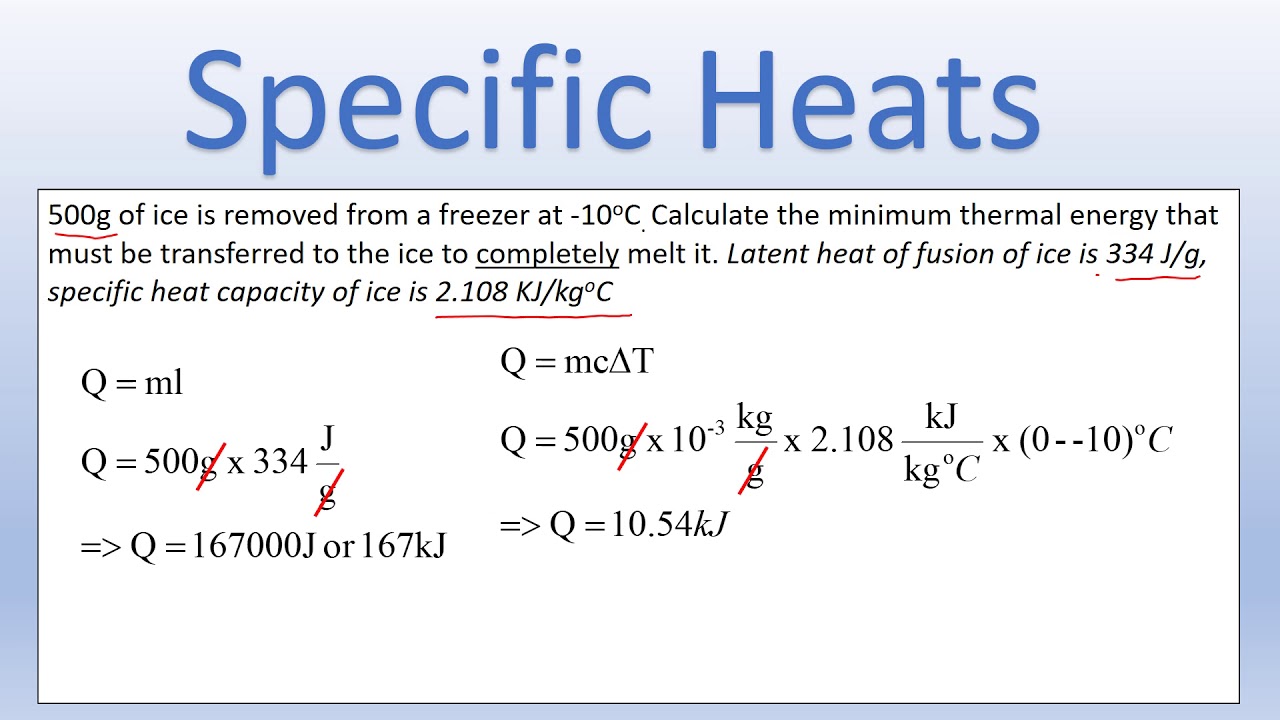
The formula for specific heat capacity is: [ q = mcΔT ]
- Calculate Unknowns: Depending on what is known:
- If (q), (m), and (ΔT) are known, you can find (c) by rearranging the formula: [ c = \frac{q}{mΔT} ]
- If (c), (m), and (ΔT) are known, calculate (q) using the original formula.
- Choose the Right Units: Ensure all units are consistent. If your values are in different units, convert them to ensure the calculation is valid.
🔍 Note: Keep an eye on units! J, kcal, BTU, and other energy units need conversion to Joules for consistency in SI units.
- Interpret Results: After calculation, interpret your results:
- Assess how the material responds to heat.
- Understand the implications for energy efficiency in practical applications like heating systems, cooling processes, or material science experiments.
Key Materials and Their Specific Heats

| Material | Specific Heat Capacity (J/g·°C) |
|---|---|
| Water | 4.186 |
| Ice | 2.093 |
| Aluminum | 0.897 |
| Copper | 0.385 |
| Iron | 0.450 |

Important Considerations

- Temperature Dependence: Specific heat can vary with temperature, so be aware that the values provided are often at standard conditions (25°C or room temperature).
- Material Impurities: Different samples of the same material might have slightly different specific heats due to impurities or structural differences.
- Phase Changes: Remember that during phase changes (e.g., melting, boiling), heat is absorbed or released without changing the temperature, altering the use of (q = mcΔT).
In summary, mastering specific heat calculations is crucial for understanding how materials respond to temperature changes, and it's applicable in a wide array of scientific and industrial processes. This guide provides a structured approach to ensuring your calculations are accurate, consistent, and insightful. As you progress, you'll gain not only a deeper appreciation for thermodynamics but also the practical skills needed to apply this knowledge effectively.
Why is specific heat important in engineering?

+
Specific heat helps engineers calculate the heat needed or energy efficiency in various systems, from engines to refrigeration units, allowing for better design and optimization.
How does specific heat affect cooking?
+
When cooking, specific heat determines how quickly food or cooking utensils heat up or cool down, affecting cooking times and thermal properties of foods.
Can specific heat capacity change with temperature?

+
Yes, it can change slightly with temperature due to changes in material structure or phase transitions. However, the change is often small enough that standard values are used for most practical applications.