5 Essential Tips for Understanding Solutes and Solvents

Understanding the concepts of solutes and solvents is fundamental in chemistry. These terms describe the components of a solution, a homogeneous mixture where one substance is dissolved in another. Here are five essential tips to help you grasp these key concepts:
1. Know What Defines a Solute and a Solvent


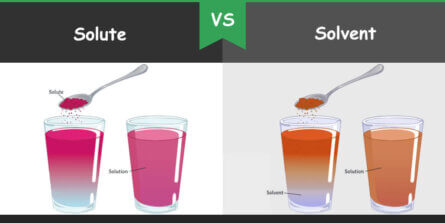
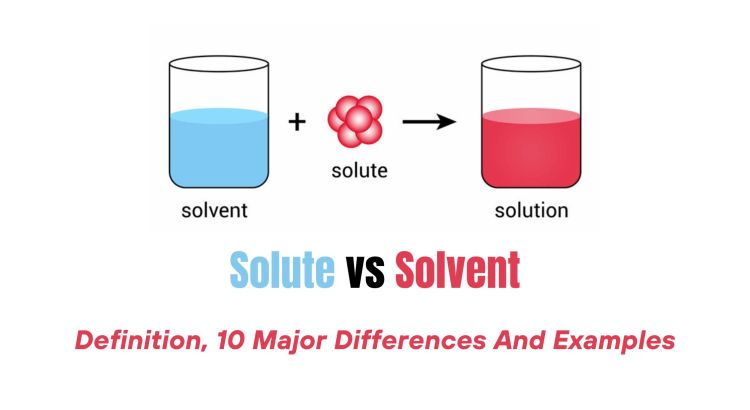
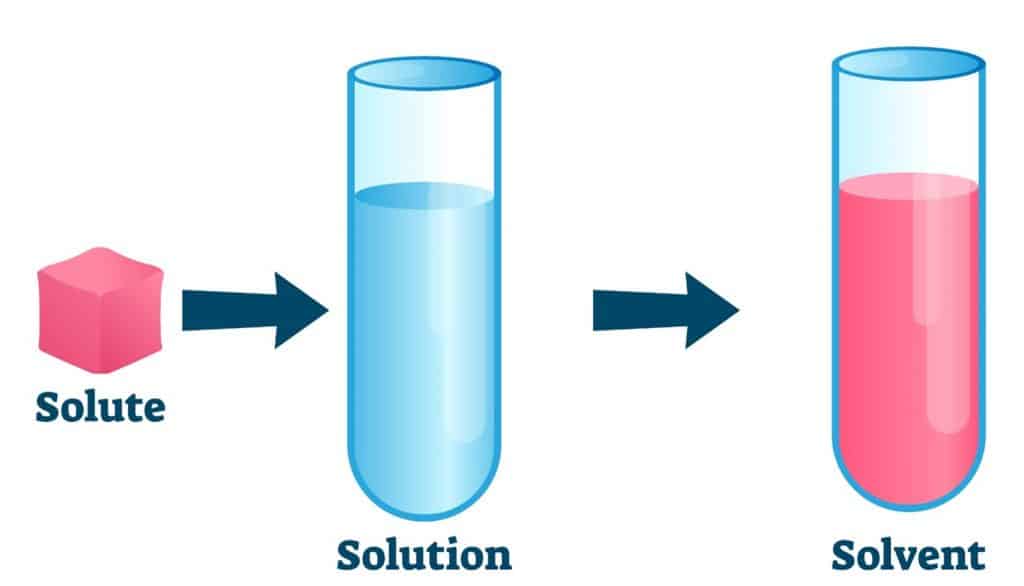
- Solute: This is the substance that gets dissolved. In any solution, the solute is the minority component.
- Solvent: The solvent does the dissolving. It’s the majority component in the solution. Common solvents include water, ethanol, and acetone.
The definition can vary with context. For example, if you dissolve sugar in water, sugar is the solute, and water is the solvent. However, if you dissolve a small amount of water in a large amount of alcohol, water becomes the solute.
2. Identify the Properties of Good Solutes and Solvents
- Solubility: Good solutes are soluble in the solvent. Solubility is influenced by factors like temperature, pressure, and the chemical nature of both solute and solvent.
- Polarity: Polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solutes. This is often explained by the rule, “Like dissolves like.”
- Chemical Compatibility: Solute and solvent must have chemical compatibility. Acidic solutes might not dissolve in basic solvents, for instance.
🧪 Note: Temperature can significantly affect solubility; some solutes dissolve better in hot solvents than in cold ones.
3. Understand the Impact of Concentration

The concentration of a solution refers to how much solute is dissolved in a solvent. Here’s how you can measure it:
| Concentration Term | Description |
|---|---|
| Molarity (M) | Moles of solute per liter of solution. |
| Molality (m) | Moles of solute per kilogram of solvent. |
| Percent Composition | Percent by mass or volume of solute in the solution. |
| Parts Per Million (PPM) | Very dilute solutions, e.g., trace amounts of contaminants in water. |

Higher concentrations lead to stronger chemical interactions between solute and solvent, which can influence the solution’s properties like boiling point and freezing point.
4. Explore Real-World Applications

Solutes and solvents are not just lab concepts; they’re vital in everyday life:
- Medical: Intravenous solutions contain salts or sugars dissolved in water.
- Industrial: Solvents like toluene or acetone are used to dissolve adhesives or paints.
- Environmental: Water pollution often involves solutes like heavy metals, chemicals, or organic matter.
🌎 Note: Solute-solvent interactions play a crucial role in the environmental fate of pollutants.
5. Practice with Experiments
Experimenting can reinforce your understanding:
- Mix sugar (solute) in water (solvent) at different temperatures to see how solubility changes.
- Observe how oil (non-polar) does not mix with water (polar), illustrating polarity effects.
- Perform titration experiments to understand how solutes react with solvents.
Engaging with practical examples provides a tangible way to see how solutes and solvents interact under different conditions.
In wrapping up, the interaction between solutes and solvents forms the backbone of many chemical processes. By understanding these interactions, you can better comprehend chemical reactions, physical properties of solutions, and the environmental impact of substances. These five tips provide a framework for not only understanding but also applying the principles of solutes and solvents in various scientific and everyday contexts.
What is the difference between a solute and a solvent?
+
A solute is the component that gets dissolved in a solution, typically present in smaller quantities. A solvent is the medium in which the solute dissolves, usually present in larger quantities.
How can one determine if a solute will dissolve in a solvent?

+
The solubility of a solute in a solvent depends on factors like chemical compatibility (polarity), temperature, pressure, and the chemical nature of the solute and solvent. Generally, polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solutes.
Why is concentration important in solutions?

+
Concentration determines the strength or intensity of the solution. It affects various properties of the solution like boiling point, freezing point, vapor pressure, and the rate of chemical reactions that can occur within the solution.
What is meant by the term “Like Dissolves Like”?

+
“Like Dissolves Like” is a rule of thumb in chemistry stating that substances with similar chemical properties tend to dissolve in each other. Polar compounds dissolve in polar solvents, and non-polar compounds dissolve in non-polar solvents.