Master Solubility Rules: Worksheet With Answers

The concept of solubility is fundamental in chemistry, impacting various fields from material science to environmental engineering and pharmacology. Understanding solubility rules aids chemists in predicting how substances will behave in solutions, which is critical for experimental design, synthesis, and industrial processes. In this post, we'll delve into the core solubility rules, provide a detailed worksheet with solutions, and discuss some common applications to enrich your grasp on this topic.
Why Are Solubility Rules Important?
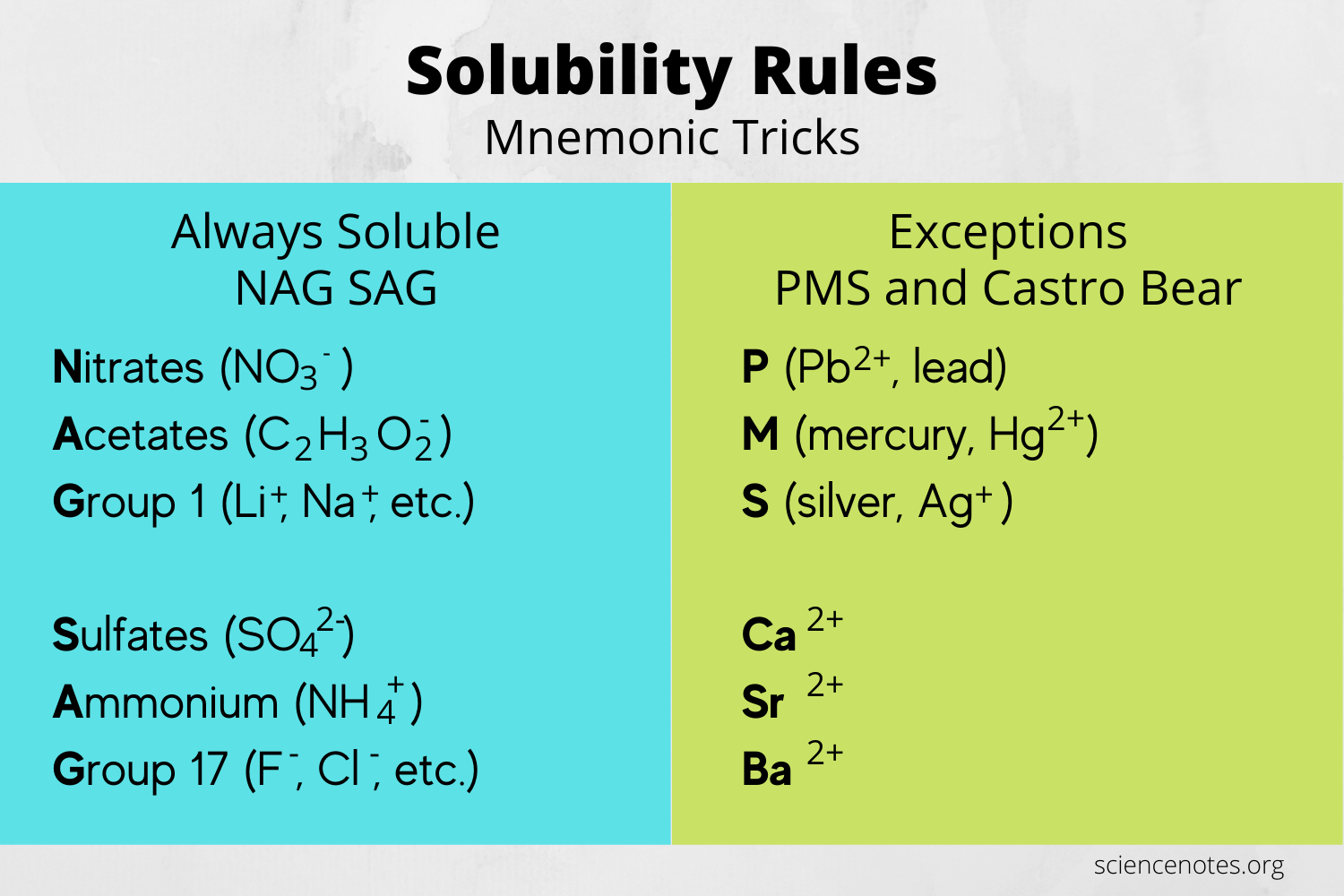
Solubility rules help chemists to:
- Predict whether a compound will dissolve in water or other solvents.
- Understand precipitation reactions, which are crucial in qualitative analysis.
- Design and optimize separation techniques like filtration and recrystallization.
- Anticipate the behavior of compounds in various environmental and biological systems.
General Solubility Rules

Here are some general solubility guidelines to keep in mind:
- Salts containing Group I elements (Li+, Na+, K+, etc.) are usually soluble.
- Ammonium salts (NH₄+) are generally soluble.
- Nitrates (NO₃-), acetates (CH₃COO-), chlorates (ClO₃-), and perchlorates (ClO₄-) are typically soluble.
- Most sulfate salts are soluble, but notable exceptions include those containing Ba2+, Sr2+, Ca2+, Pb2+, and Ag+.
- Most chlorides (Cl-), bromides (Br-), and iodides (I-) are soluble. The primary exceptions are those with Ag+, Pb2+, and Hg₂²⁺.
- Carbonates (CO₃²⁻), phosphates (PO₄³⁻), sulfides (S²⁻), and silicates are mostly insoluble, with the exception of those with Group I and ammonium ions.
Worksheet with Answers
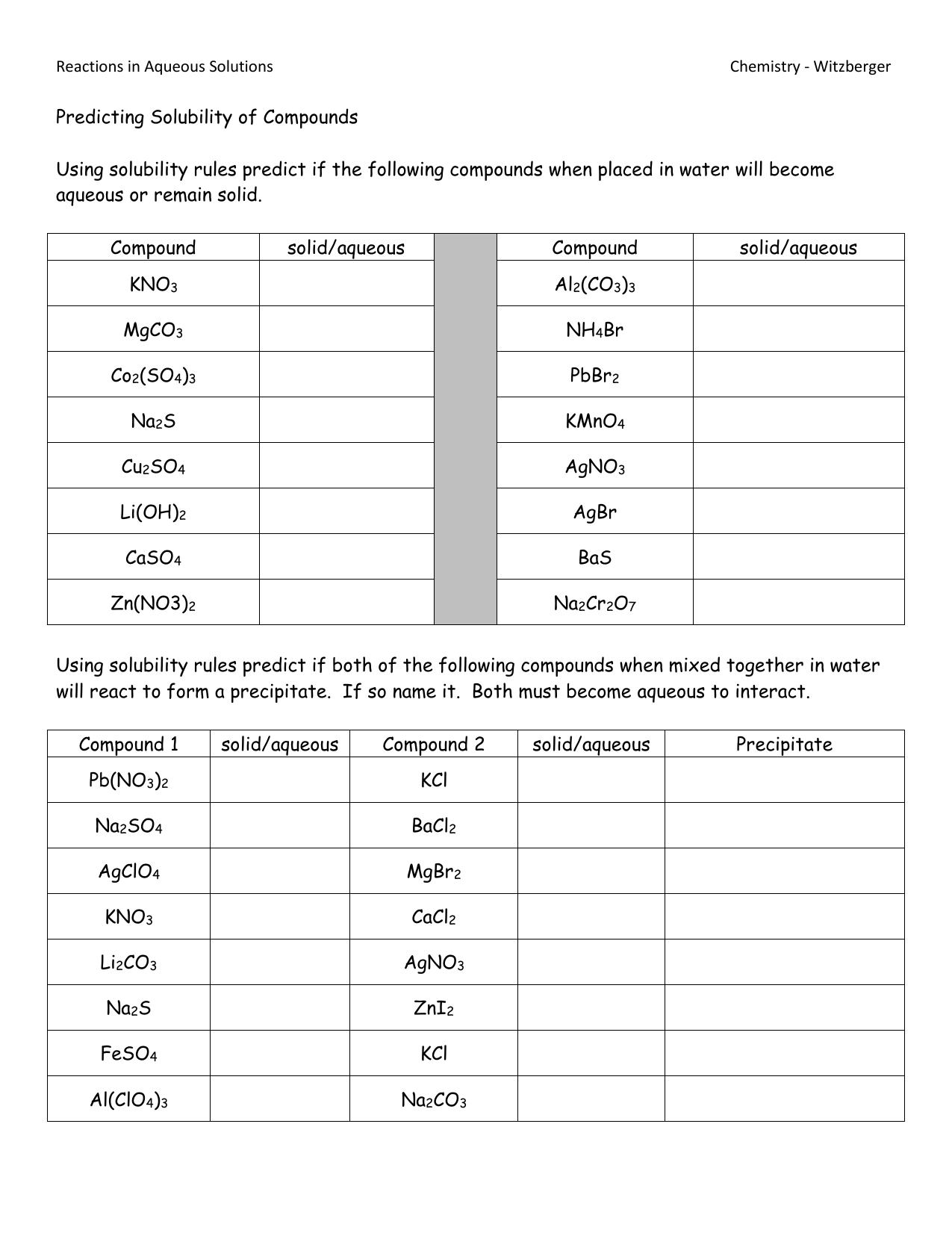
Here's a worksheet to test your understanding of solubility rules:
| Question | Answer |
|---|---|
| Is K₂SO₄ soluble or insoluble? | Soluble (contains Group I elements) |
| Will PbCl₂ precipitate in water? | Yes, lead chloride is not soluble (exception to the chloride rule) |
| What happens when sodium acetate (CH₃COONa) is added to water? | It will dissolve (acetates are soluble) |
| Is CaCO₃ soluble? | No, it is largely insoluble (carbonates are generally insoluble except with Group I elements) |
| Which of these are soluble: Na₂CO₃, MgSO₄, AgCl, BaCO₃? | Na₂CO₃, MgSO₄ (Na₂CO₃ because it's a Group I carbonate, MgSO₄ because it's an exception to the sulfate solubility rule) |

💡 Note: Remember that solubility rules provide generalizations; actual solubility can be influenced by concentration, temperature, and pH.
Common Applications of Solubility Rules

The solubility rules are not just academic exercises; they have practical applications in:
- Water treatment: Precipitation of contaminants to purify water.
- Pharmaceutical manufacturing: Ensuring drug compounds dissolve properly or precipitate out as required.
- Environmental monitoring: Assessing solubility to predict the movement of pollutants in soil and water.
- Metallurgy: Extracting metals from ores by leaching or precipitation.
Summary

Solubility rules are essential tools for understanding chemical behavior in solutions. They guide predictions about solubility, facilitate reactions in experiments, and are integral in various industries for process optimization. The worksheet above illustrates how these rules can be applied practically, showing that mastering them not only enhances theoretical knowledge but also equips you to handle real-world chemical scenarios.
What makes a compound soluble?

+
A compound is generally considered soluble if it can dissolve in a solvent, usually water, forming a homogeneous solution. The solubility is influenced by the nature of the solute and solvent, the forces between their molecules or ions, and the energy required to break and form these bonds.
Can solubility rules change with different solvents?

+
Yes, solubility rules primarily apply to water as a solvent. Different solvents have different polarity, dielectric constants, and chemical interactions, leading to different solubility behaviors for the same compounds.
How does temperature affect solubility?

+
Temperature can significantly influence solubility. Generally, the solubility of most solids increases with an increase in temperature, allowing more solute to dissolve in the same volume of solvent. Conversely, the solubility of gases usually decreases with rising temperature because gas molecules gain kinetic energy and escape the solution more easily.
Why are some compounds exceptions to the solubility rules?

+
Exceptions often occur due to specific ion or molecular interactions, the size and charge density of ions, or complexation and lattice energy considerations. For instance, silver chloride (AgCl) is insoluble despite chlorides being generally soluble because Ag+ forms a stable, low solubility complex with Cl-.