Solubility Rules Chem Worksheet 15 1 Answer Key

Solubility rules are essential tools for chemists, providing a framework to predict whether substances will dissolve in water, forming aqueous solutions, or remain undissolved as precipitates. For students and professionals alike, understanding these rules can streamline experimental procedures, enhance predictive modeling, and facilitate better understanding of chemical behavior in various reactions. Let's delve into the intricacies of the Solubility Rules Chem Worksheet 15-1, which offers a hands-on approach to mastering these vital concepts.
What are Solubility Rules?
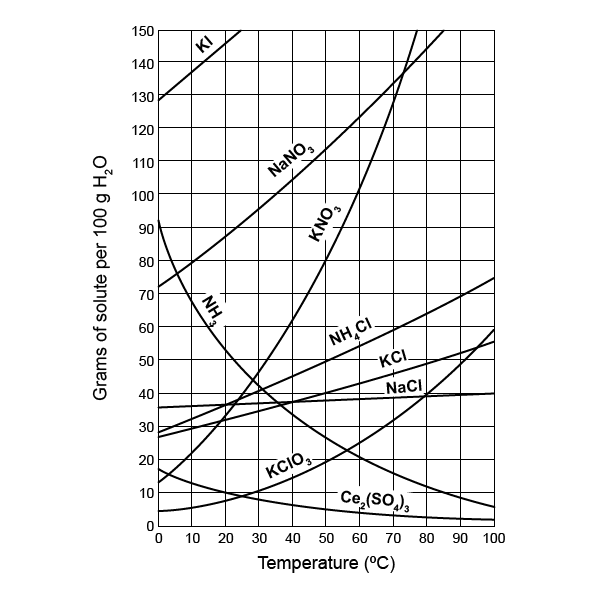
Solubility rules help us determine the solubility of salts, acids, and bases in water. Here are some foundational principles:
- Salts containing Group I elements (Li+, Na+, K+, etc.) are usually soluble.
- Ammonium salts (NH4+) are also typically soluble.
- Nitrates (NO3-) and acetates (CH3COO-) are soluble with very few exceptions.
- Chlorides, bromides, and iodides (Cl-, Br-, I-) are soluble, except with Ag+, Pb2+, and Hg22+.
- Sulfates (SO42-) are generally soluble except with calcium, strontium, barium, and lead.
- Most hydroxides (OH-) and carbonates (CO32-) are insoluble, with Group I elements and ammonium being exceptions.
The Solubility Rules Chem Worksheet 15-1

The worksheet is structured to help students apply solubility rules to practical problems:
- Section 1: Basic Rules Identification - Here, students are presented with a series of ionic compounds and must determine their solubility by applying the rules.
- Section 2: Exceptions - Focuses on identifying when exceptions to these rules occur, which is crucial for avoiding misinterpretations.
- Section 3: Mixed Solubility - Introduces more complex scenarios where ions can form both soluble and insoluble products, pushing the application of rules further.
| Compound | Solubility (Yes/No) | Rule Applied |
|---|---|---|
| NaCl | Yes | Group I elements are soluble |
| AgCl | No | Ag+ forms an insoluble chloride |
| CaCO3 | No | Most carbonates are insoluble |

These rules are not only useful in laboratory settings but also in predicting outcomes in environmental and industrial chemistry.
💡 Note: Remember that solubility is not an either-or situation. It's a spectrum, and solubility rules are generalized guidelines. Factors like temperature and pressure can significantly affect solubility.
How to Use Solubility Rules Effectively
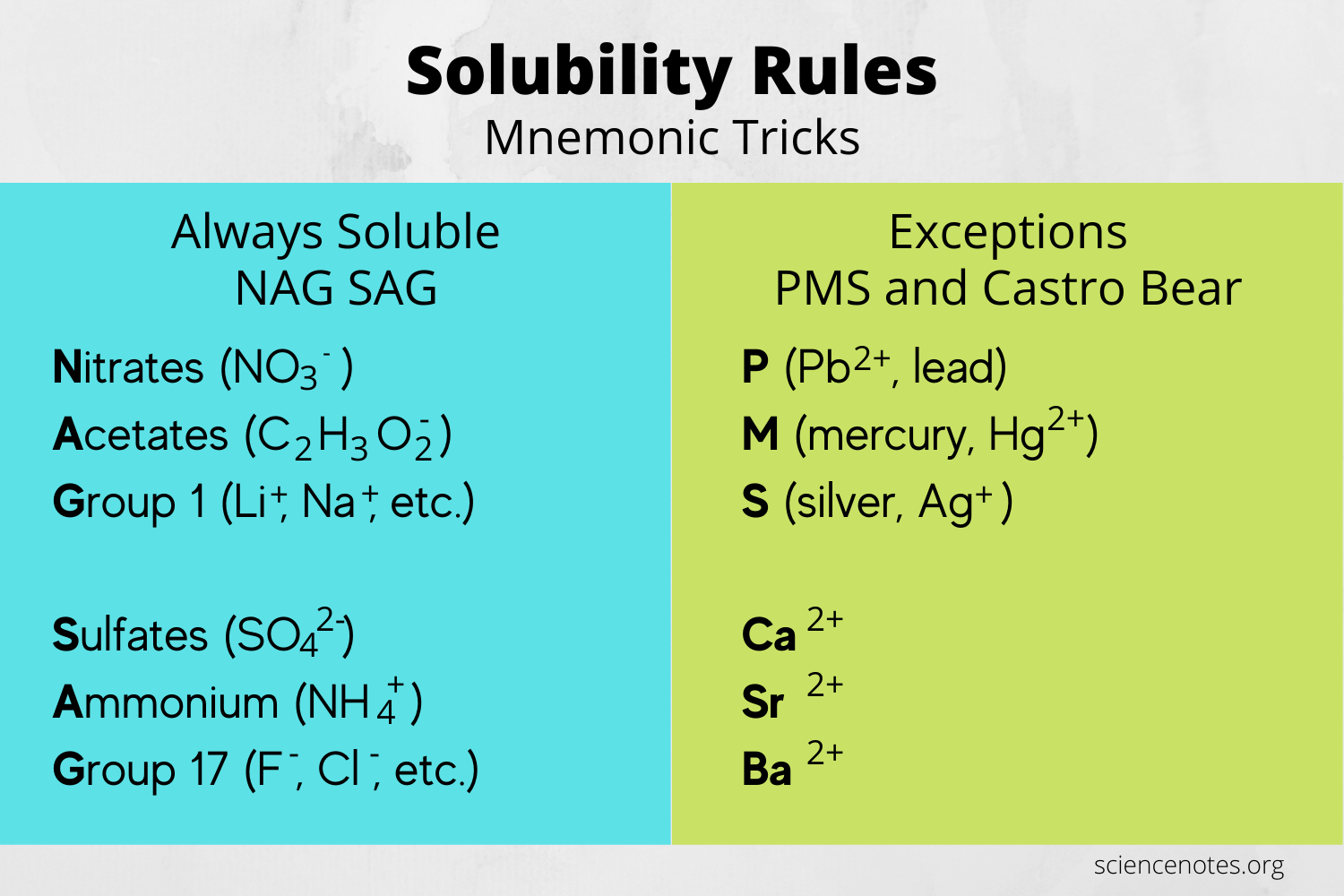
To maximize the utility of solubility rules:
- Memorize Key Rules: Start with the most common ions and their solubility behaviors.
- Apply Hierarchically: When in doubt, consider exceptions after checking general solubility.
- Understand Limitations: While useful, these rules are guidelines. Actual solubility might vary under different conditions.
⚗️ Note: Solubility rules are derived from observations and are subject to exceptions due to variations in chemical composition, temperature, and sometimes even the concentration of the ions involved.
Why Solubility Rules Matter

Understanding solubility rules is crucial for several reasons:
- Precipitation Reactions: Knowing which compounds will precipitate helps in identifying reactants and products in reactions.
- Separation Techniques: Chemists use solubility to separate mixtures based on the solubility of different compounds.
- Environmental Impact: Solubility affects how chemicals interact with the environment, from water pollution to soil contamination.
- Pharmaceutical Production: Drugs must be soluble enough to be absorbed but not so soluble that they break down too quickly.
By integrating these rules into your chemical thinking, you can:
- Streamline your experimental design by anticipating what reactions will produce precipitates.
- Optimize processes in industry, such as wastewater treatment and material synthesis.
- Enhance your ability to predict and control reactions, making your work in the lab more efficient.
To encapsulate, Solubility Rules Chem Worksheet 15-1 serves as an excellent starting point for understanding how different substances behave in solution. It's a practical guide that demystifies the complexity of chemical solubility, making it more accessible for students to grasp and apply in real-world scenarios. By mastering these rules, chemists can not only predict outcomes but also innovate in their experimental and industrial applications, leveraging the fundamental principles of solubility to advance scientific and technological frontiers.
Why are some salts soluble while others are not?

+
The solubility of salts depends on the strength of the ionic bonds within the compound and the enthalpy change associated with dissolving. Strongly ionic bonds resist breaking apart, making the compound less soluble, whereas compounds with weaker ionic bonds or favorable solvation energies are more likely to dissolve.
What factors can influence the solubility of a compound?

+
Temperature is a significant factor, with many salts becoming more soluble as temperature increases. Pressure affects gases dissolved in liquids, but less so for salts. The nature of the solvent, ionic size, and polarity also play crucial roles in solubility.
Are there any universal exceptions to solubility rules?
+
While solubility rules are widely applicable, exceptions do exist due to unique chemical interactions. For instance, barium hydroxide is slightly soluble, though most hydroxides are not. Always refer to specific solubility tables for detailed information.