Solubility: Polar vs Nonpolar Worksheet Answers Explained
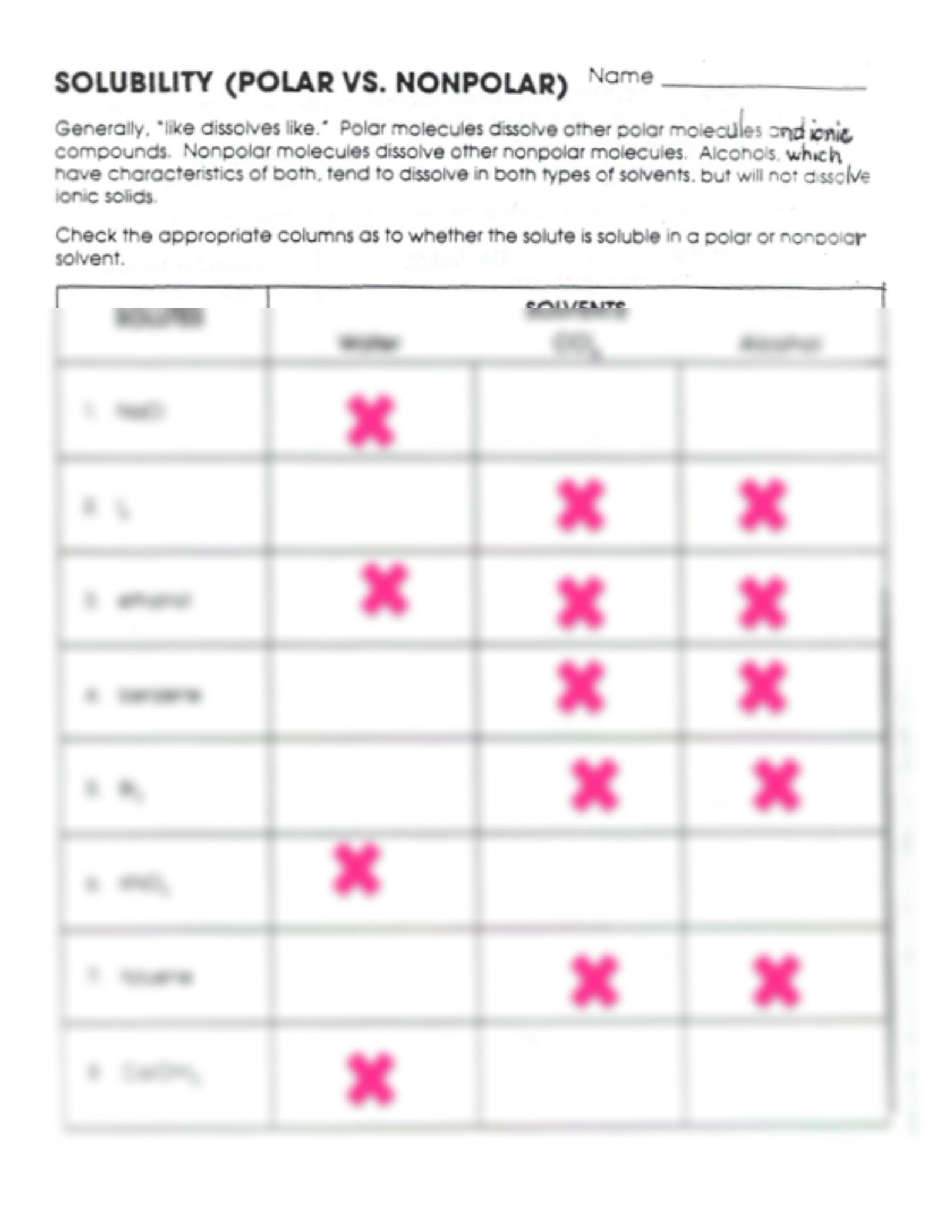
The realm of chemistry often introduces the fundamental concept of solubility, where substances dissolve in solvents, often with an emphasis on polarity. Understanding why some compounds mix while others repel can be quite intriguing. Today, let's explore how polarity affects solubility, a key element in various chemical processes.
Understanding Solubility

Solubility refers to the maximum amount of solute that can dissolve in a solvent at equilibrium. This is typically measured in grams of solute per liter of solvent. Here are some key points:
- Like dissolves like: Polar solvents tend to dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
- Solubility can be influenced by temperature, pressure, and chemical composition of both the solute and solvent.
Polarity and Its Effect on Solubility
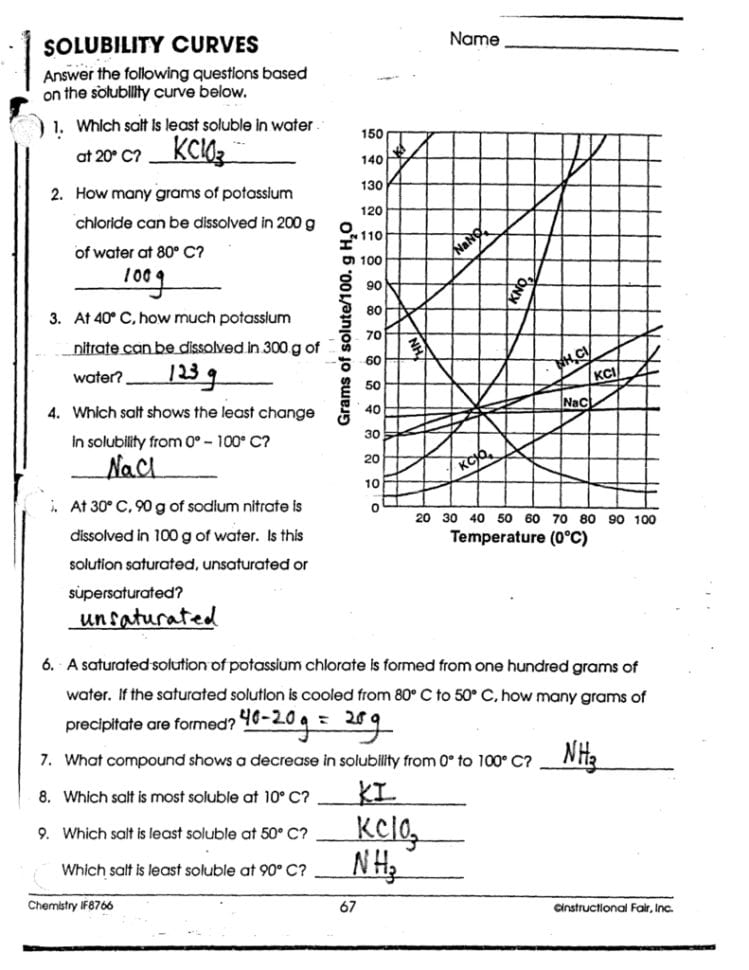
Polarity in a molecule arises from an uneven distribution of electrons, leading to areas of partial positive and negative charges. Here’s how polarity impacts solubility:
- Polar Solvents: Water (H2O), alcohols, and ammonia (NH3) are common examples. They have a dipole moment due to electronegative atoms attracting electrons more strongly.
- Nonpolar Solvents: Hexane, toluene, and diethyl ether are typical. These solvents lack significant dipole moments due to similar electronegativity of bonded atoms.
| Solvent | Polarity | Examples of Solutes Dissolved |
|---|---|---|
| Water (H2O) | Polar | Salt (NaCl), Sugar (C12H22O11), Ethanol (C2H5OH) |
| Hexane (C6H14) | Nonpolar | Oils, greases, nonpolar hydrocarbons |
| Ethanol (C2H5OH) | Polar | Water, Acetone (CH3COCH3), Sodium Chloride (NaCl) |

The key to understanding solubility involves the interaction between solute-solvent forces. Polar solvents dissolve polar solutes because:
- The solvent's partial charges can interact with the solute's charges, reducing the energy required to dissolve the solute.
- Hydrogen bonding, a special dipole-dipole force, plays a significant role in polar solubility.
Conversely, nonpolar solvents dissolve nonpolar solutes due to:
- The absence of significant charge separation in both solvent and solute, leading to London dispersion forces (van der Waals forces) as the primary intermolecular interaction.
⚗️ Note: The dissolution process is an interplay of enthalpy and entropy. While the enthalpy (heat energy) required to break bonds between solute and solvent molecules can predict solubility to some extent, entropy (the disorder or randomness of the system) also contributes significantly.
In summary, understanding the solubility of polar and nonpolar substances involves recognizing the molecular interactions, both within and between the solute and solvent. The term "like dissolves like" serves as a guiding principle in predicting solubility outcomes.
Why does oil not dissolve in water?

+
Oil, being nonpolar, does not mix with water, a polar solvent, due to the lack of attraction between their molecules. Water molecules prefer to bond with each other through hydrogen bonds, creating a barrier for oil molecules.
Can a substance be soluble in both polar and nonpolar solvents?

+
Yes, some substances like alcohols (e.g., ethanol) can dissolve in both polar and nonpolar solvents because they possess both polar (hydroxyl group) and nonpolar (alkyl chain) regions.
What is the importance of understanding solubility in chemistry?
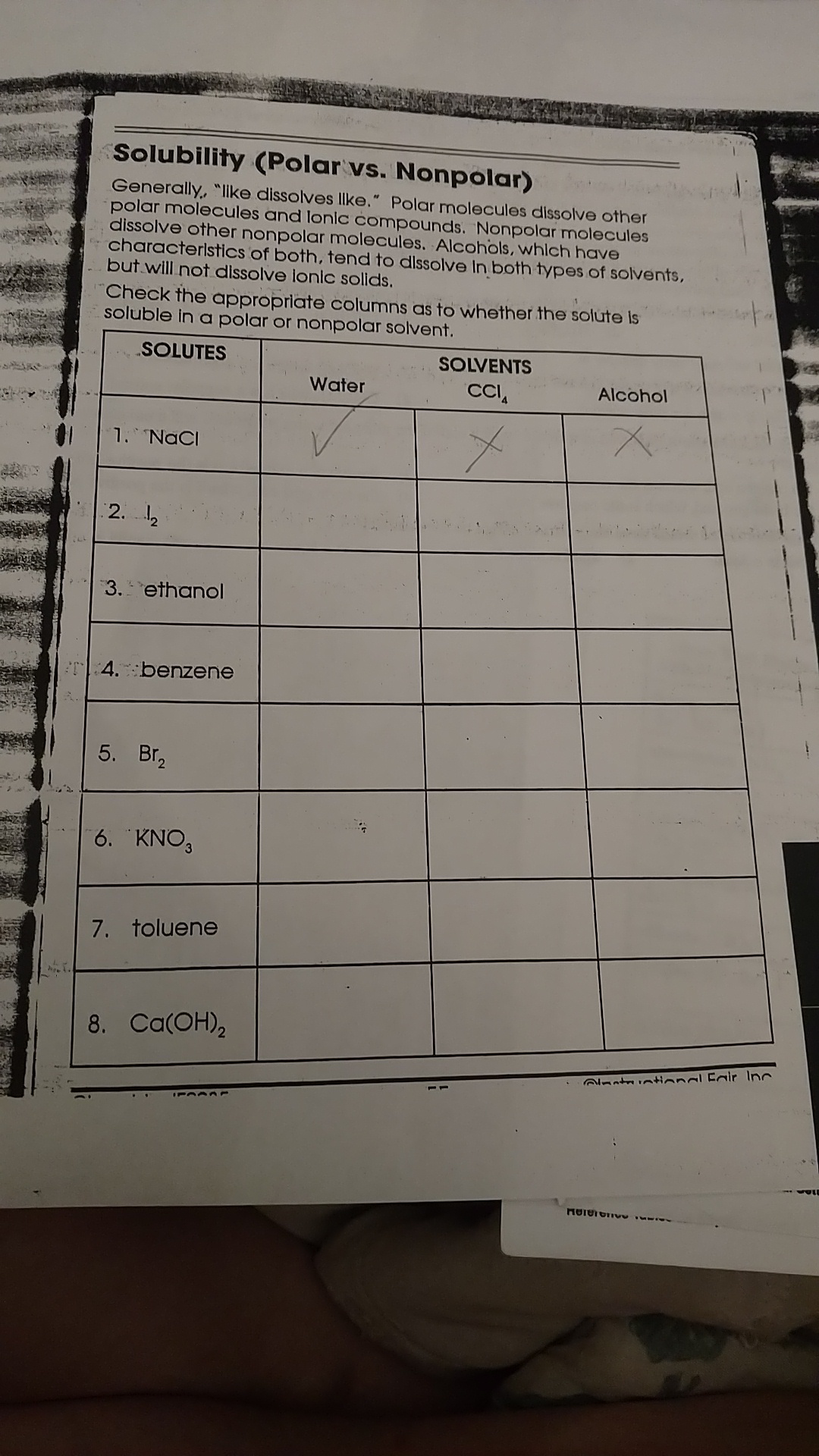
+
Understanding solubility is crucial for applications in drug formulation, environmental science, cooking, and many industrial processes where mixing substances effectively is required.