Unlocking Solubility Graphs: Worksheet Answers Revealed

Understanding solubility graphs can be a fascinating journey into the realm of chemistry, where visual representations help us predict how substances dissolve in solvents, particularly water. These graphs are essential tools for students, scientists, and enthusiasts looking to delve deeper into the solubility of various compounds. In this comprehensive guide, we will demystify solubility graphs by analyzing various worksheets and providing detailed answers, thereby unveiling the secrets behind solubility behaviors.
Solubility Graphs: An Introduction

Solubility is defined as the maximum amount of a solute that can dissolve in a solvent at a given temperature. Solubility graphs, therefore, plot solubility versus temperature, offering insights into:
- How temperature affects solubility
- Identifying solubility trends
- Predicting when precipitation might occur
Understanding the Axes

Before diving into worksheet examples, it’s crucial to understand the components of a solubility graph:
- Y-Axis: Represents the solubility in grams of solute per 100 grams of solvent (usually water).
- X-Axis: Represents temperature in degrees Celsius (°C).
🌡️ Note: Solubility can either increase or decrease with temperature, depending on the solute.
Sample Worksheet Analysis
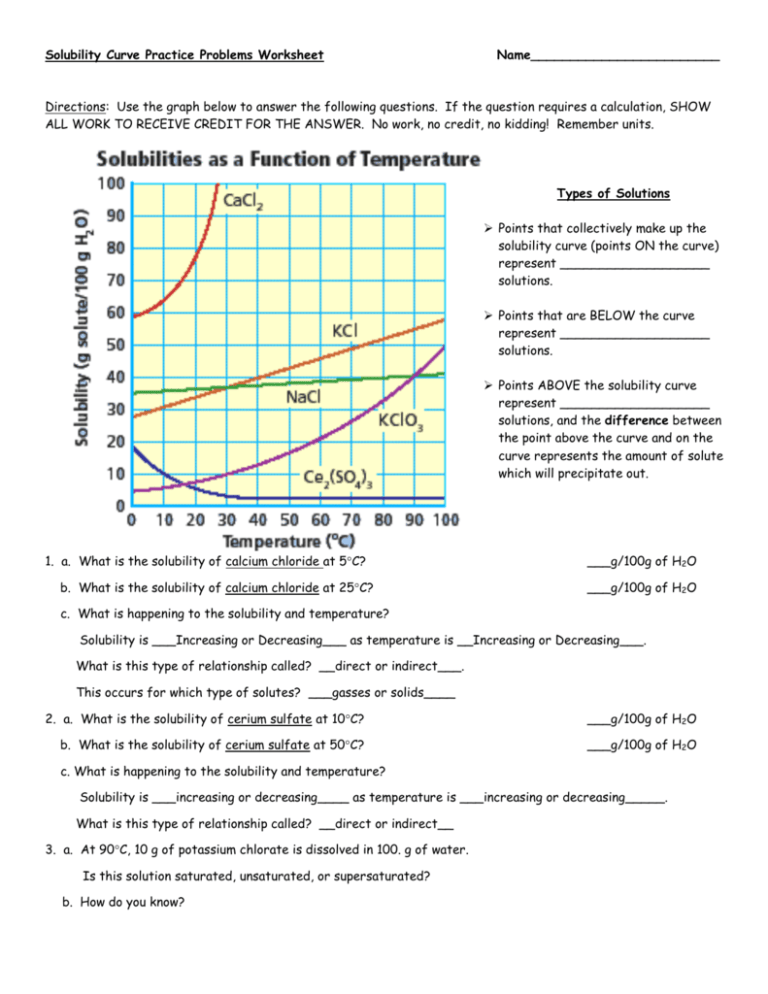
Let’s walk through a common worksheet scenario involving different salts to enhance our understanding of solubility graphs.
Worksheet Example 1: Solubility of Potassium Nitrate (KNO3)

The worksheet provides data points for the solubility of KNO3 at various temperatures. Here is how we interpret this information:
| Temperature (°C) | Solubility (g/100 mL H2O) |
|---|---|
| 0 | 13 |
| 20 | 31 |
| 40 | 51 |
| 60 | 73 |
| 80 | 96 |
| 100 | 120 |
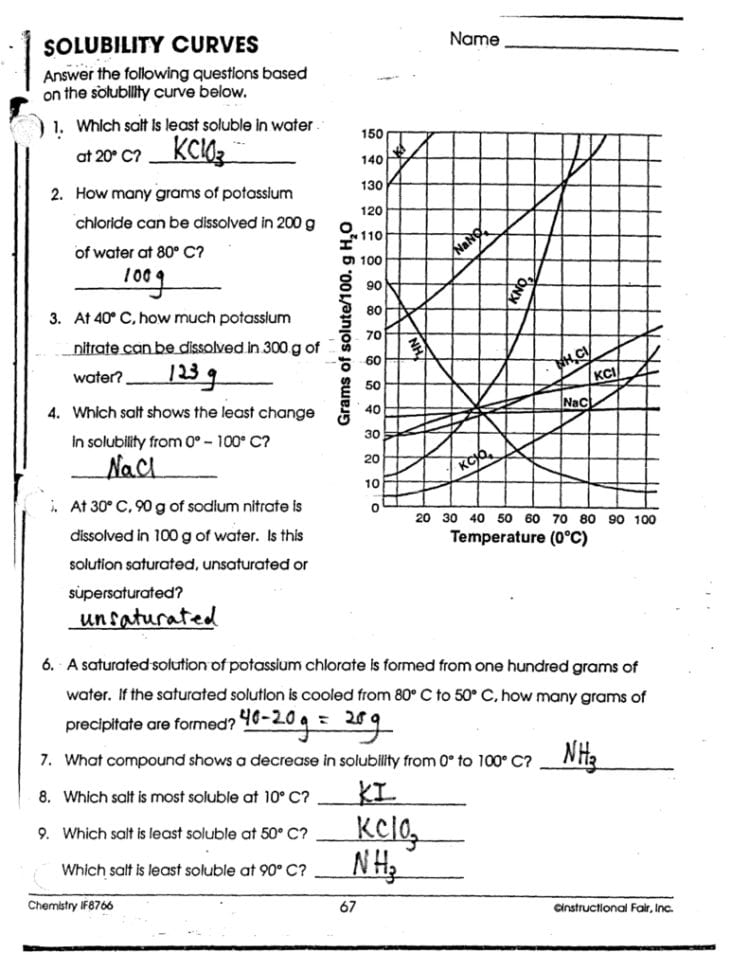
From the table, we can draw several conclusions:
- The solubility of KNO3 increases as temperature rises, indicating a positive slope on the solubility graph.
- Between 0°C and 100°C, the solubility more than triples, showcasing the significant effect temperature has on this compound’s solubility.
Worksheet Example 2: Multiple Salts

Another worksheet might ask to compare the solubility behavior of several salts like sodium chloride (NaCl), calcium chloride (CaCl2), and potassium sulfate (K2SO4):
| Compound | 0°C (g/100mL) | 20°C (g/100mL) | 60°C (g/100mL) |
|---|---|---|---|
| NaCl | 35.7 | 35.9 | 37.1 |
| CaCl2 | 59.5 | 64.7 | 127.8 |
| K2SO4 | 11.1 | 13.9 | 31.5 |
Analyzing these data points:
- NaCl shows minimal change in solubility with temperature increase, suggesting temperature has little effect on its solubility.
- CaCl2 exhibits a sharp increase in solubility as temperature rises, similar to KNO3.
- K2SO4 demonstrates a moderate increase in solubility with temperature, but not as dramatic as CaCl2 or KNO3.
Unveiling the Answers

To answer the questions typically found in solubility graph worksheets:
Question 1: What is the solubility of Potassium Nitrate at 50°C?

Using the provided data or estimating from the graph:
At 50°C, KNO3 has a solubility of approximately 61 grams per 100 mL of water.
Question 2: Which Salt Exhibits the Least Solubility Change with Temperature?

From the analysis above, sodium chloride (NaCl) shows the least solubility change with temperature, making it the answer to this question.
Question 3: At What Temperature Does CaCl2 Begin to Precipitate?

If you had a saturated solution of CaCl2 at 60°C and then cooled it, you would observe:
The solubility of CaCl2 at 60°C is 127.8 grams per 100 mL. As the solution cools, the solubility decreases, and precipitation would begin when the solution temperature drops below 60°C and reaches the solubility limit at that lower temperature.
To Summarize

In conclusion, solubility graphs are invaluable for predicting how much of a substance can dissolve in a given solvent at specific temperatures. Through our examination of potassium nitrate, sodium chloride, calcium chloride, and potassium sulfate, we’ve uncovered not only the fascinating world of solubility but also how temperature influences it. These insights help in understanding and controlling processes in fields from chemical engineering to pharmaceuticals and beyond.
Why do some salts show increased solubility with temperature while others do not?

+
The behavior of salt solubility with temperature depends on the energy required to break the solute-solute bonds (enthalpy of solution) versus the energy gained from forming solvent-solute interactions. If breaking the solute bonds requires more energy than is provided by solvent-solute interactions, solubility increases with temperature (endothermic process). Conversely, if the dissolution is exothermic, solubility might not increase as significantly or may even decrease.
How can solubility graphs help in preventing crystallization?

+
By understanding the solubility-temperature relationship, one can maintain the solution at a temperature where the solute remains dissolved. For example, keeping a solution above its precipitation point to avoid crystal formation or managing temperature changes to control crystal growth in industrial processes.
What role does pressure play in solubility graphs?

+
For gases dissolving in liquids, Henry’s Law states that solubility is proportional to the partial pressure of the gas. However, for solids in liquids, pressure has minimal effect on solubility, hence it’s not typically considered in standard solubility graphs.
Related Terms:
- Solubility graphs worksheet answers pdf
- Solubility graph Worksheet PDF
- Solubility curve Worksheet
- Solubility curve questions and answers