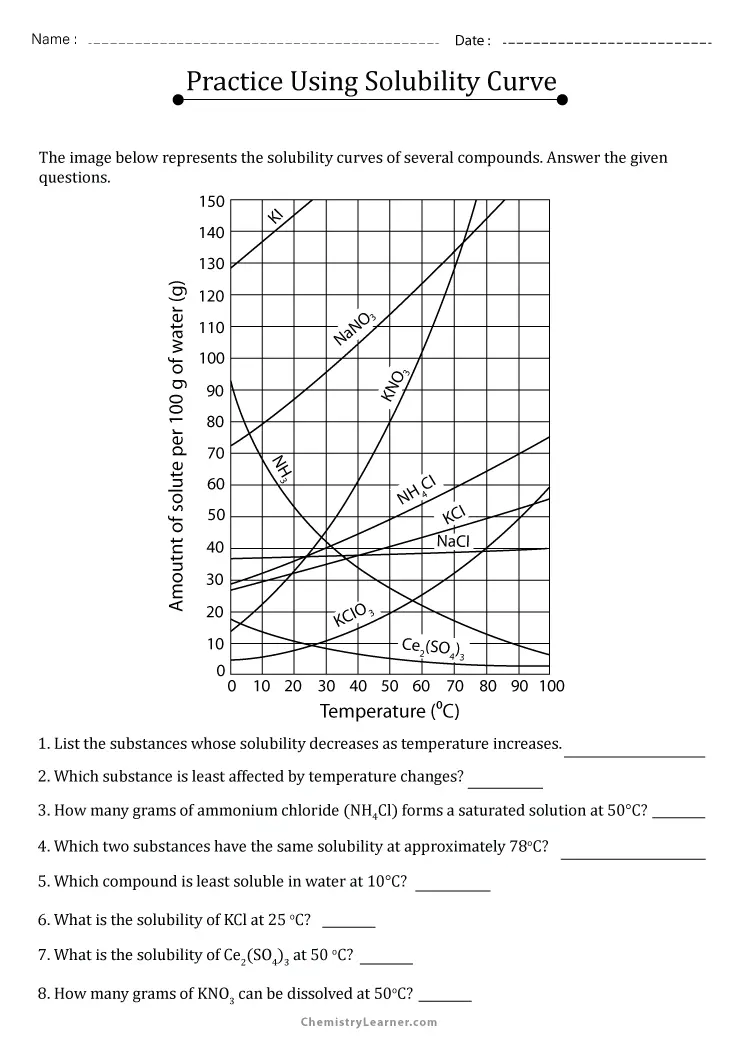
Master Solubility Curves with Our Interactive Worksheet 2

Solubility curves are an essential tool in chemistry, especially when studying solutions and mixtures. These curves visually represent the amount of solute that can dissolve in a given solvent at different temperatures. If you're looking to master understanding and interpreting these curves, this interactive worksheet is designed just for you.
Understanding Solubility Curves

Solubility refers to the maximum amount of solute that will dissolve in a solvent at a given temperature, forming a saturated solution. Here's what you need to know:
- Saturated Solution: A solution where no more solute can dissolve at that temperature.
- Unsaturated Solution: A solution that contains less solute than can dissolve at a given temperature.
- Supersaturated Solution: A solution that contains more solute than it should normally hold at a given temperature, often formed by cooling a saturated solution.
⚗️ Note: Solubility increases with temperature for most substances. Water, for example, can dissolve more sugar at higher temperatures.
How to Use Solubility Curves

Solubility curves provide a wealth of information:
- Reading the Curve: On a solubility curve, the horizontal axis represents temperature, and the vertical axis shows the amount of solute that can dissolve in 100 grams of water.
- Interpretation: By following the curve, you can determine how much solute dissolves at a specific temperature. Points above the curve indicate supersaturation, while points below indicate unsaturation.
Here is a sample solubility curve:
| Temperature (°C) | Grams of Solute per 100 grams of Water |
|---|---|
| 0 | 35.8 |
| 20 | 39.1 |
| 40 | 42.2 |
| 60 | 45.4 |
| 80 | 48.7 |
| 100 | 52.0 |

Interactive Worksheet for Learning

Our interactive solubility curve worksheet includes exercises that guide you through the following steps:
- Reading Solubility Data: Learn how to interpret the data from the solubility curve.
- Calculating Solubility: Use the curve to calculate the solubility of different solutes at various temperatures.
- Predicting Solubility: Predict how much solute is needed to achieve saturation at a given temperature.
- Comparing Solubilities: Compare the solubility behavior of different solutes.
Each exercise is accompanied by questions and interactive tools to:
- Drag and drop solubility data points onto a curve.
- Use sliders to change temperature and observe changes in solubility.
- Answer fill-in-the-blank questions based on the solubility curves.
Practical Applications
Understanding solubility curves isn't just for academics; it has real-world applications:
- Pharmaceuticals: Drug formulation and dissolution rates.
- Environmental Science: Pollution and contaminant solubility.
- Food Science: Food preservation and processing techniques.
- Chemical Engineering: Process optimization and waste management.
🔍 Note: In solubility studies, the term 'like dissolves like' often applies, where polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solutes.
Conclusion

In summary, solubility curves are crucial for understanding how substances behave in solution, and mastering them can open doors to a variety of scientific disciplines. Our interactive worksheet serves as an engaging tool to learn and apply solubility concepts practically, enhancing both comprehension and retention. Whether for academic purposes or real-life problem-solving, these curves are indispensable in the study of chemistry.
What does the saturation point on a solubility curve represent?

+
The saturation point on a solubility curve represents the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature. Beyond this point, any additional solute will not dissolve.
Can solubility decrease with temperature?
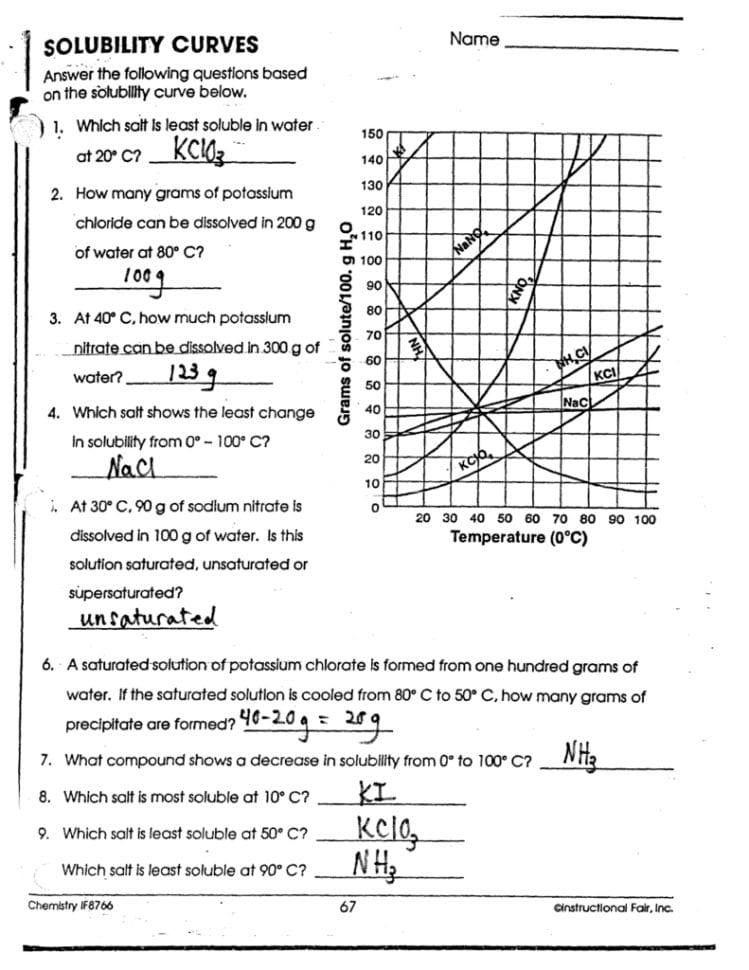
+
Yes, though rare, some substances like gas solutes (e.g., oxygen or carbon dioxide in water) have solubility that decreases as the temperature increases.
How does pressure affect solubility?
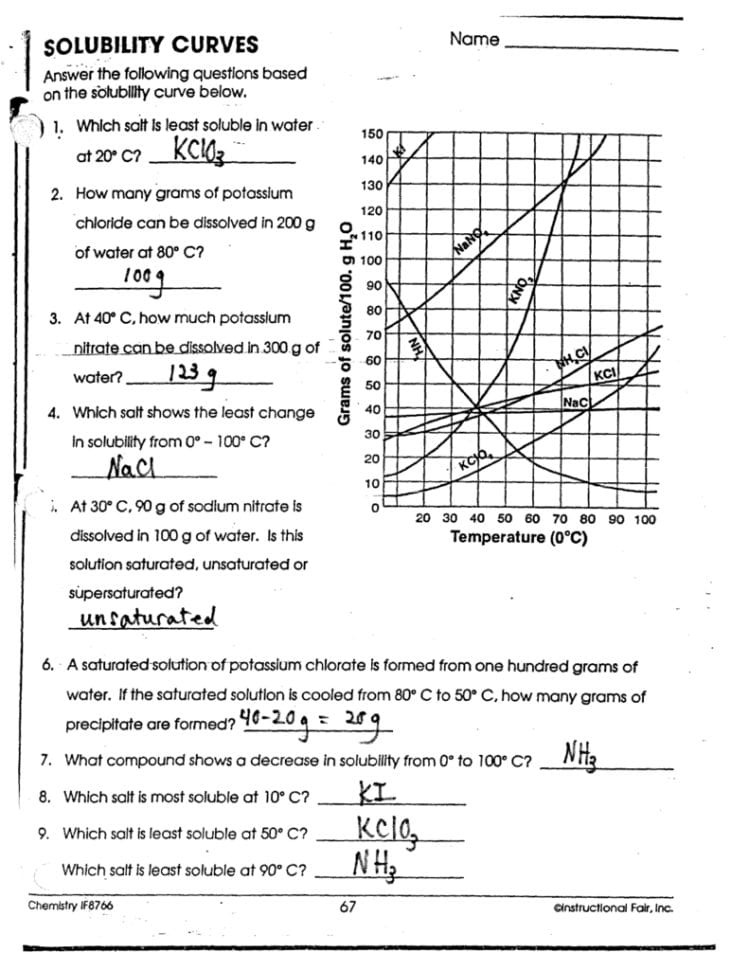
+
Pressure primarily affects the solubility of gases in liquids. According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the pressure of that gas above the liquid. Solids and liquids are less affected by pressure changes.



