Solubility Curves Worksheet: Mastering Chemistry Solutions

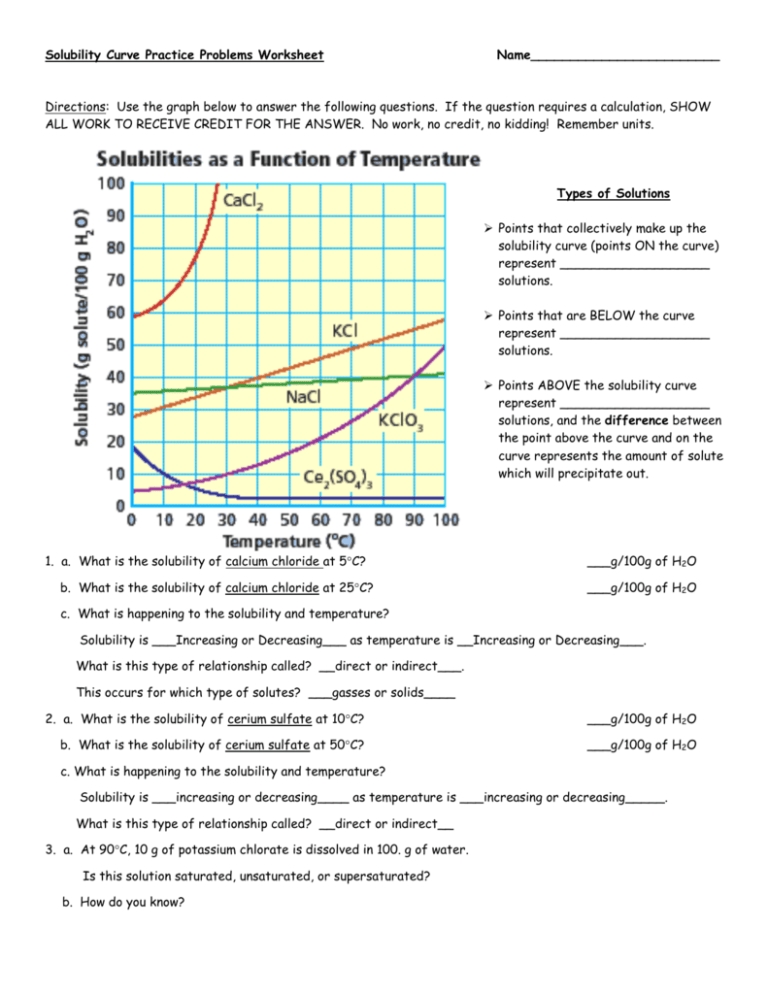
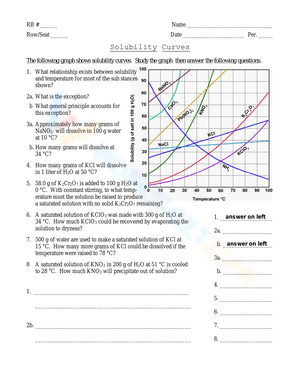
In the fascinating world of chemistry, solubility curves play a pivotal role in understanding how various substances dissolve in solvents. This knowledge isn't just theoretical; it has practical applications in fields ranging from pharmaceuticals to environmental science. For students and enthusiasts, diving into the intricacies of solubility curves provides a window into predicting how much solute can be dissolved in a solvent at different temperatures. This mastery of chemistry solutions is essential for both academic success and real-world applications.
What Are Solubility Curves?

Solubility curves are graphical representations that show the amount of a solute that can dissolve in a given volume of solvent at various temperatures. Typically, these curves are plotted with temperature on the x-axis and solubility in grams per 100 grams of solvent on the y-axis.
Understanding the Curve

- Temperature Dependency: Most substances increase in solubility as temperature increases. This is due to the increased kinetic energy of the solvent molecules, which allows them to break solute-solute interactions more effectively.
- Maximum Solubility: At a given temperature, there’s a maximum amount of solute that can dissolve. This point on the solubility curve indicates the saturation point.

🧪 Note: The shape of solubility curves can differ significantly between different solutes, reflecting their unique chemical behavior.
Why Solubility Curves Matter

Understanding solubility curves allows:
- Chemists to predict how much solute will dissolve at various temperatures.
- Researchers to design processes that involve dissolving substances, like in crystallization techniques.
- Environmental scientists to assess the impact of pollutants dissolving in natural bodies of water.
Working with Solubility Curves
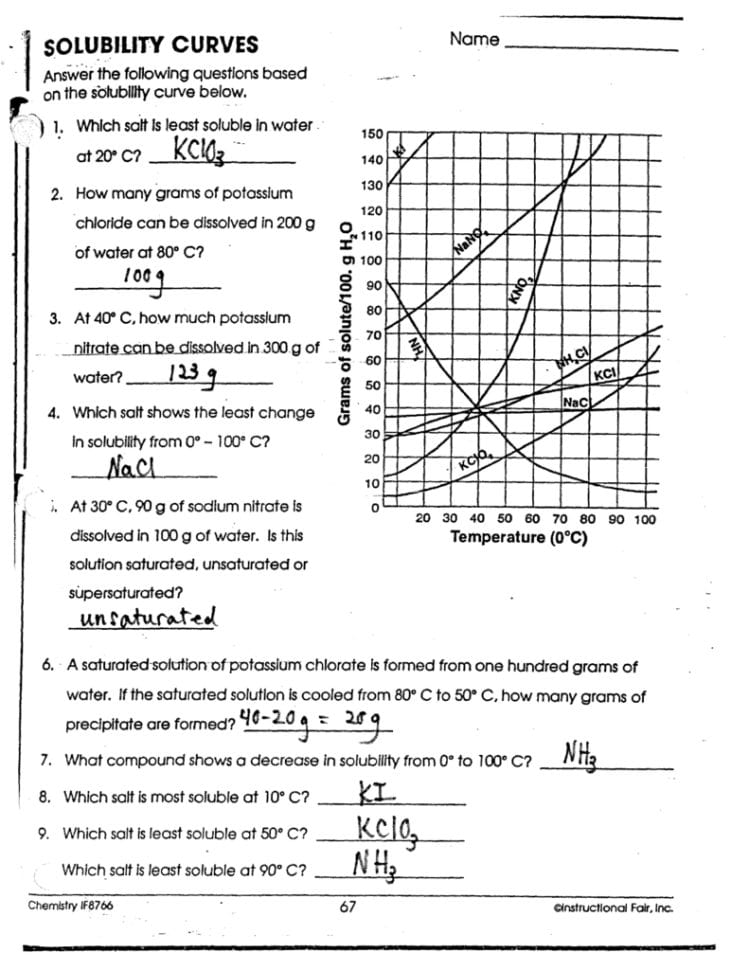
To use solubility curves effectively:
- Read the Curve: Locate the temperature on the x-axis, then follow it up to where it intersects the curve for the solute in question.
- Interpret the Data: The y-axis value at the point of intersection indicates the solubility at that specific temperature.
- Compare Different Solutes: By comparing the curves of different solutes, one can understand their relative solubility behaviors.
Practical Exercise

Here’s a practical worksheet to help you master solubility curves:
| Solute | Solubility at 20°C (g/100g H₂O) | Solubility at 60°C (g/100g H₂O) | Solubility Change (g/100g H₂O) |
|---|---|---|---|
| Sodium Chloride (NaCl) | 35.7 | 37.0 | 1.3 |
| Potassium Nitrate (KNO₃) | 31.6 | 101.0 | 69.4 |
| Glucose (C₆H₁₂O₆) | 91.0 | 110.0 | 19.0 |

🔍 Note: Using solubility curves can help identify supersaturated solutions, where more solute is dissolved than is typically possible at a given temperature.
Applications of Solubility Curves

Mastering solubility curves has real-world implications:
- Chemical Synthesis: Chemists can determine optimal conditions for reactions involving dissolution.
- Food Science: Solubility affects the taste, texture, and shelf life of many food products.
- Pharmaceutical Industry: Drug formulation relies heavily on understanding solubility for effective delivery systems.
By exploring how different solutes dissolve, students and professionals can predict and control chemical reactions, manipulate substances' physical properties, and solve practical problems in various industries.
Having navigated through the intricacies of solubility curves, it's clear that they are not just lines on a graph but tools for understanding and manipulating the physical world. They empower us to foresee the behavior of solutes in solvents under different thermal conditions, enhancing our capability in science and technology.
Whether you're looking to refine a synthesis process, understand environmental impacts, or just to pass a chemistry exam, mastering solubility curves is an indispensable part of learning and applying chemistry. This journey from mere data points to actionable insights reflects the true spirit of scientific inquiry and application.
Why do solubility curves slope upward for most substances?

+
Most substances show an increase in solubility with temperature because the kinetic energy of solvent molecules increases, allowing them to disrupt solute-solute interactions more effectively, thus increasing solubility.
How does solubility affect drug formulation in the pharmaceutical industry?

+
Understanding solubility helps in formulating drugs to ensure they dissolve at the right rate and in the correct concentration in the body. This is crucial for bioavailability, efficacy, and reducing side effects.
Can solubility curves predict supersaturation?
+
Yes, solubility curves can help identify when a solution might become supersaturated, as they show the maximum amount of solute that can dissolve at a given temperature. Exceeding this limit indicates supersaturation.
What can we learn from comparing the solubility curves of different substances?

+
Comparing solubility curves reveals how different solutes interact with solvents at various temperatures, providing insights into their chemical behavior and suitability for specific applications or conditions.
How is solubility relevant in environmental science?
+
Solubility determines how pollutants like fertilizers, pesticides, or industrial chemicals disperse into bodies of water, influencing ecosystem health, cleanup strategies, and regulatory policies.