5 Ways to Master Solubility Curves with Answers
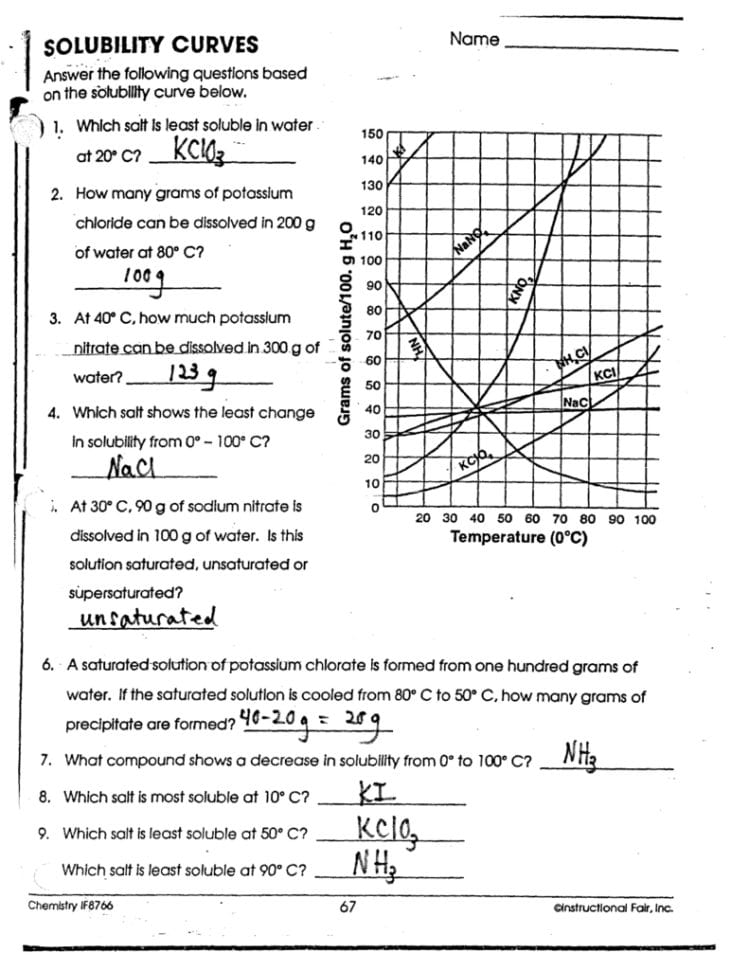
Understanding solubility curves is crucial for students and professionals in chemistry. These curves provide insights into how much solute can dissolve in a solvent at different temperatures. Mastering solubility curves not only aids in theoretical knowledge but also in practical applications like crystal growth, pharmaceutical manufacturing, and environmental science. Here are five detailed strategies to master solubility curves effectively.
1. Understand the Basics of Solubility
Before diving into the complexities of solubility curves, one must grasp the fundamental concept of solubility:
- What is Solubility? Solubility refers to the amount of a substance (solute) that can dissolve in a specific amount of another substance (solvent) under given conditions.
- Units of Solubility: It’s usually expressed in grams of solute per 100 grams of solvent at a given temperature.
- Solubility Curves: A graphical representation where the solubility of the solute versus temperature is plotted, showing how solubility changes with temperature.

2. Study the Curve Patterns

Recognizing patterns in solubility curves helps in understanding and predicting solubility behavior:
- Positive Slope: Most solids exhibit an increase in solubility with an increase in temperature, leading to a positive slope on the solubility curve.
- Negative Slope: Some substances, like calcium sulfate, show decreasing solubility with rising temperature, resulting in a negative slope.
- Curvature: Curves might not be perfectly straight; some show pronounced curvatures indicating complex solubility-temperature relationships.
3. Experiment with Solubility

Practical experiments reinforce theoretical learning:
- Conducting Tests: Prepare solutions with different temperatures, and observe how much solute dissolves. Compare with known solubility data.
- Record Keeping: Maintain a lab notebook with your observations, including temperatures, solute masses, and dissolution times.

4. Use Mathematical Models

Solubility can often be expressed using mathematical models:
- Equations: Use formulas like the van’t Hoff equation to understand how temperature affects solubility.
- Regression Analysis: Fit solubility data to a polynomial or other suitable function to predict solubility at different temperatures.
| Solvent | Solute | Temperature (°C) | Solubility (g/100g of H2O) |
|---|---|---|---|
| Water | Nacl | 0 | 35.7 |
| Water | Nacl | 100 | 39.8 |

5. Analyze Real-World Applications

Connect theoretical knowledge with real-world scenarios:
- Pharmaceutical Industry: Understanding solubility helps in drug formulation and ensuring drugs dissolve properly in the body.
- Environmental Engineering: Solubility curves can predict pollutant behavior in water bodies or soil.
- Food Science: Solubility influences the flavor profiles and physical properties of food products.
In mastering solubility curves, it's important to consider both the theoretical principles and their practical applications. These strategies, from understanding basic principles to analyzing real-world applications, provide a robust framework for anyone looking to excel in the study of solubility:
🔍 Note: Regular practice with different solutes and solvents at various temperatures can deepen your understanding of solubility curves.
Why do some solubility curves show a decrease in solubility with increasing temperature?
+
This phenomenon occurs when the dissolution process is exothermic, meaning heat is released. As temperature increases, the solubility decreases because adding heat would shift the equilibrium towards less solute dissolution to counteract the temperature increase.
How can one predict solubility without performing experiments?

+
With known solubility data, one can use mathematical models like the van’t Hoff equation or linear regression to predict solubility at different temperatures.
What role does pressure play in solubility curves?
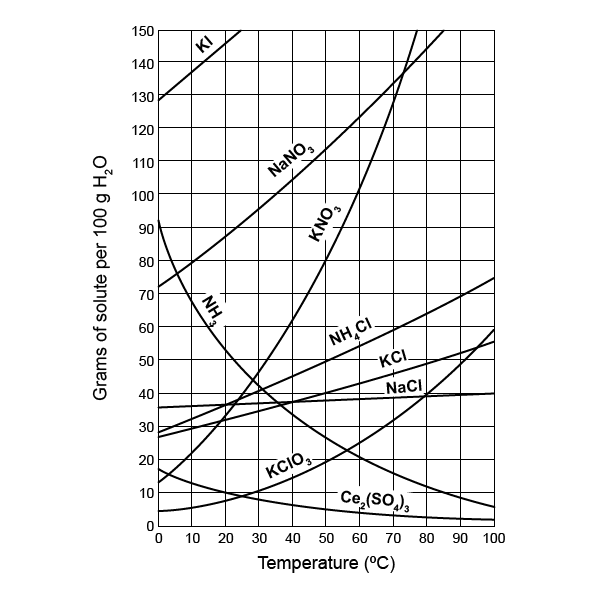
+
Pressure primarily affects the solubility of gases in liquids. Henry’s Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid. For solids, pressure has a negligible effect.



