5 Key Tips for Mastering Solubility Curve Problems
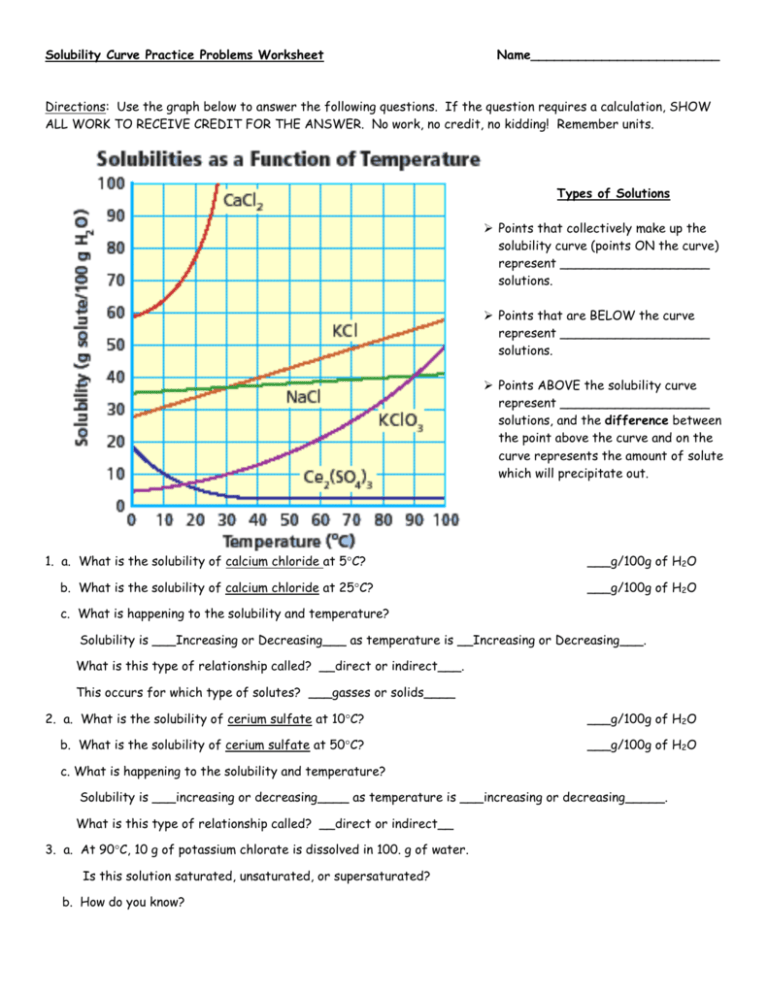
Solubility curve problems are often an integral part of chemistry courses, offering students a visual representation of how the solubility of various substances changes with temperature. Mastering these problems not only improves one's understanding of solubility but also enhances analytical skills, which are crucial in various scientific and industrial fields. Here, we present five key tips to effectively tackle solubility curve problems.
1. Understand the Basics of Solubility Curves

A solubility curve is a graph that plots the temperature on the x-axis and the solubility (usually in grams of solute per 100 grams of solvent) on the y-axis. Here's what you need to know:
- Upward Slope: Most solids become more soluble with increasing temperature, hence the graph shows an upward trend. For example, you'll find that the solubility of sodium chloride (NaCl) in water increases as the temperature rises.
- Saturation Point: This curve indicates when a solution is saturated at various temperatures. Points above the curve suggest supersaturation, while points below indicate unsaturated solutions.
🌟 Note: Understanding solubility trends helps predict how changes in temperature will affect solubility.
2. Interpret Data Correctly

Reading solubility curves involves interpreting the graph to answer questions about solubility:
- At a Given Temperature: To find the solubility at a specific temperature, draw a vertical line up from the x-axis (temperature) to the curve, then draw a horizontal line to the y-axis to read the solubility.
- Comparing Substances: If multiple substances are plotted, you can easily compare their solubility by observing the intersection of curves at different temperatures.
📊 Note: Accurate interpretation requires attention to the scale on both axes.
3. Solve for Supersaturation and Undersaturation
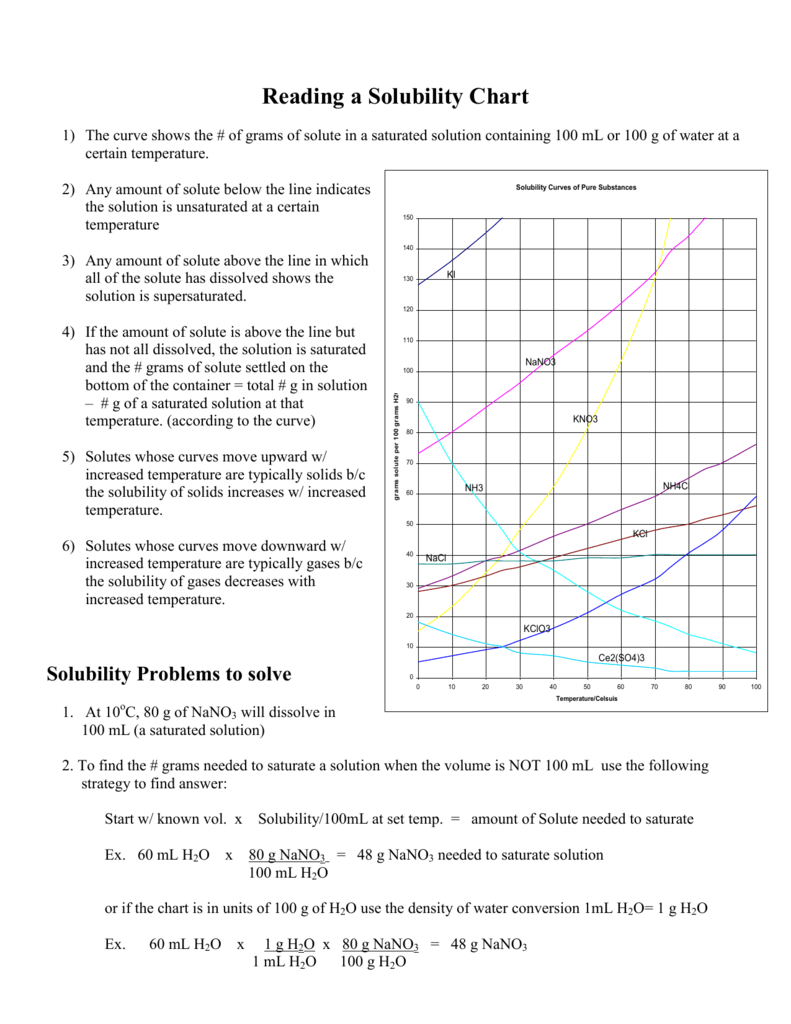
The position relative to the solubility curve will help you determine:
| Position | State |
|---|---|
| Above Curve | Supersaturated |
| On Curve | Saturated |
| Below Curve | Unsaturated |
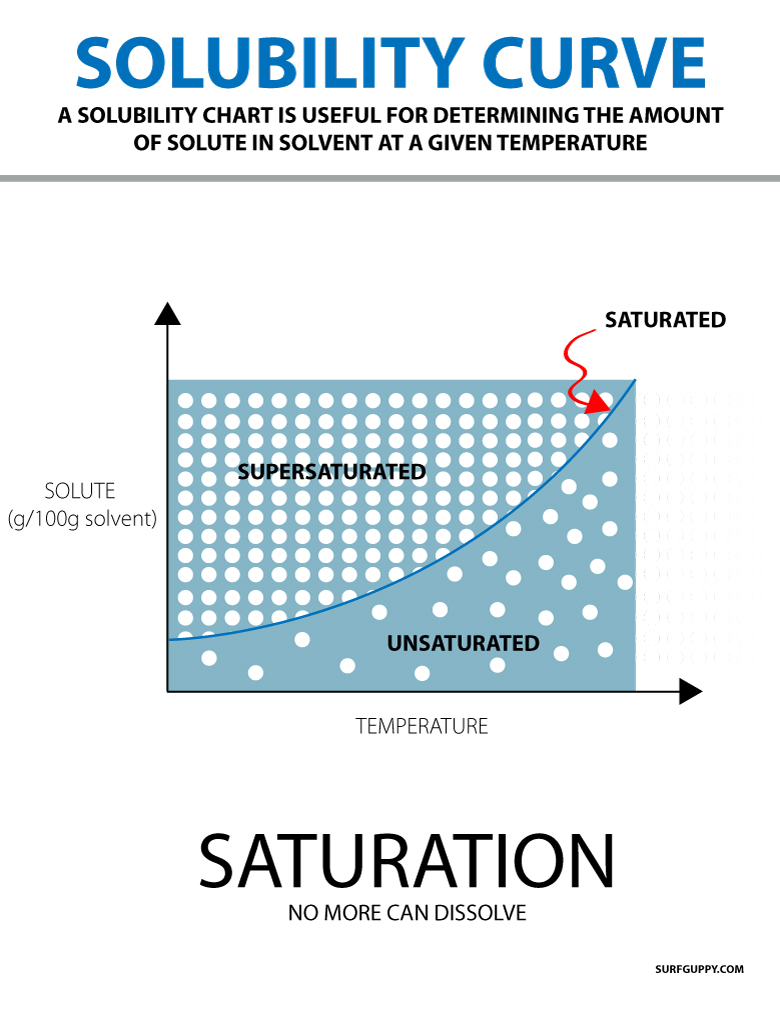
4. Use Solubility Curves in Calculations

Solubility curves can be utilized in various calculations:
- Mass of Solute: Given the volume of solvent and the temperature, use the solubility curve to determine the maximum mass of solute that can dissolve.
- Recrystallization: For purifying compounds, cooling a hot saturated solution slowly allows for the solute to come out of solution as crystals, which you can predict using solubility curves.
⚗️ Note: Calculations often involve assumptions that the solubility does not change at the boundaries of temperature increments shown on the curve.
5. Practice With Real-World Applications
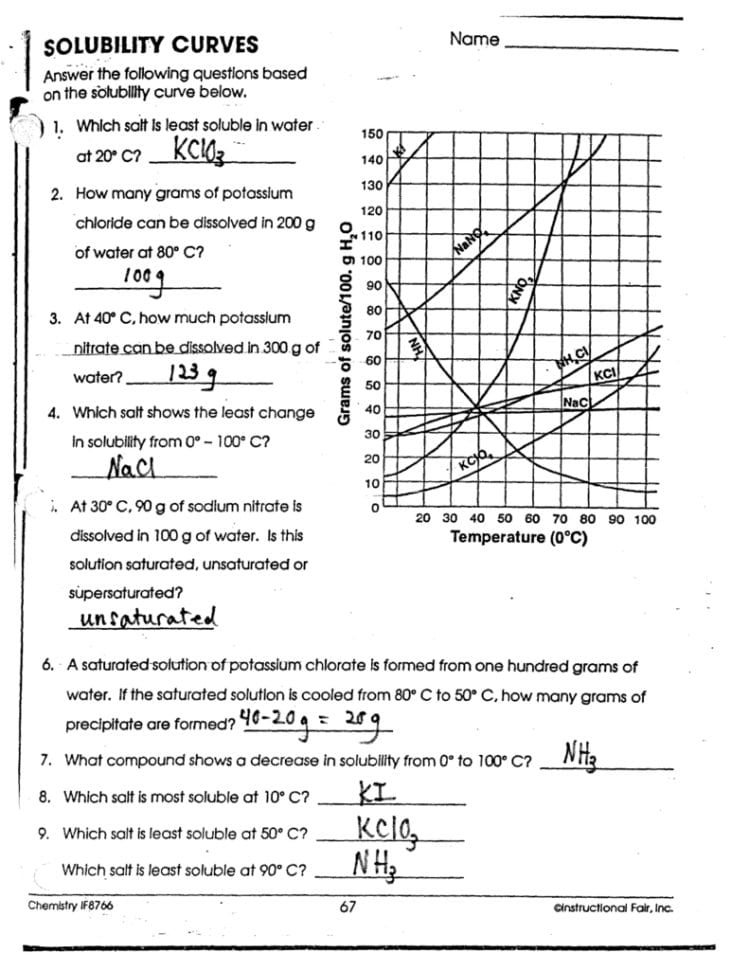
To really master solubility curve problems, apply them to real-world scenarios:
- Pharmaceutical Production: Understanding how drugs dissolve in the body at different temperatures can affect dosage forms and administration methods.
- Environmental Science: Solubility curves are used to predict how pollutants will dissolve in water bodies, affecting cleanup strategies.
🌍 Note: The ability to interpret solubility curves is critical for problem-solving in various scientific disciplines.
In wrapping up, we recognize that mastering solubility curve problems involves a deep understanding of chemical solubility principles, accurate data interpretation, and practical applications. By embracing these five key tips, you'll not only be able to solve textbook problems with ease but also apply these skills in real-world scenarios where solubility is crucial. Whether it's understanding pharmaceutical formulations or environmental contamination, these insights give you the tools to excel in chemistry and related fields.
What is the significance of a solubility curve in chemistry?

+
Solubility curves provide a graphical representation of how the solubility of a substance changes with temperature, which is essential for determining the conditions under which substances will dissolve, crystallize, or precipitate.
Can you have a solution above the solubility curve?

+
Yes, a solution can exist above its solubility curve, which means it is supersaturated. However, this is a metastable state, and the solute might precipitate out if the solution is disturbed or if crystallization is nucleated.
How does temperature affect solubility?

+
For most solids, solubility increases with temperature due to increased kinetic energy allowing the solute particles to more effectively interact with the solvent. However, there are exceptions, like certain salts or gases where solubility decreases as temperature rises.
What are some practical applications of solubility curves?
+
Solubility curves are used in various fields including pharmaceuticals for drug formulation, environmental science for pollution control, chemical engineering for crystallization processes, and even in food science for optimizing the crystallization of sugar in syrups.