Solubility Curve Worksheet: Solve Problems Easily

Understanding solubility curves is a fundamental aspect of chemistry that students often encounter in their studies. These curves graphically represent how the solubility of a substance changes with temperature, offering a wealth of information about the solubility behaviors of various compounds. This blog post is dedicated to simplifying the process of solving problems related to solubility curves, providing a clear, step-by-step guide for students.
Understanding Solubility Curves
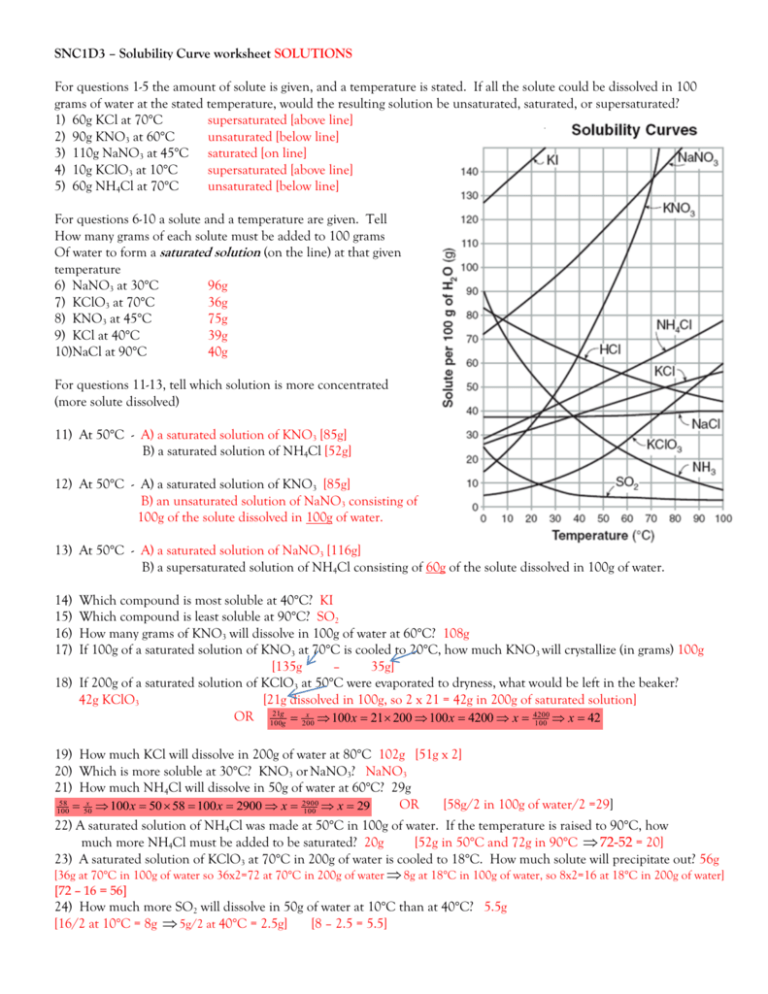
Solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. Solubility curves are line graphs where:
- The vertical axis typically represents the solubility (in grams of solute per 100 grams of solvent).
- The horizontal axis represents the temperature (usually in degrees Celsius).
Image of a Solubility Curve
How to Use Solubility Curves

Here’s how to read and interpret solubility curves:
- Identify the Substance: Each curve on the graph corresponds to a different substance.
- Determine Temperature: Find the temperature on the x-axis.
- Read Solubility: Go vertically from the temperature to the solubility curve for the substance, then read the solubility value on the y-axis.
- Compare Solubility: At a given temperature, compare how different substances dissolve in the solvent.
💡 Note: Not all substances increase in solubility with temperature; some decrease, like sodium sulfate.
Solving Common Solubility Problems

Let’s address typical problems students might solve using solubility curves:
Problem 1: Maximum Solubility

To find how much of a substance can dissolve at a specific temperature:
- Choose your substance from the graph.
- Find the temperature.
- Read the solubility at that temperature.
Problem 2: Temperature for Specific Solubility

If you need to know the temperature at which a solute has a specific solubility:
- Look for the solubility value on the y-axis.
- Trace horizontally to the curve for the substance.
- Read the temperature where the line meets the curve.
Problem 3: Saturation and Supersaturation
Understanding whether a solution is saturated or supersaturated:
- Saturated solutions are at the solubility curve; any undissolved solute will remain in equilibrium with the solution.
- Supersaturated solutions occur above the solubility curve; they are unstable and can precipitate.
Tips for Solving Problems Efficiently

- Use a ruler or a straight edge when reading values from the graph for accuracy.
- Check the scale: Ensure you’re not mistaking milliliters for grams or vice versa.
- Understand trends: Knowing if a substance’s solubility increases or decreases with temperature can help in quick approximations.
Common Mistakes and How to Avoid Them
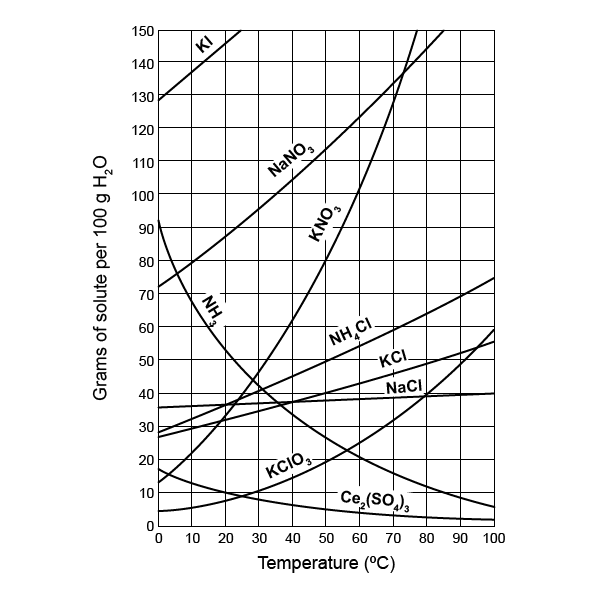
- Reading the wrong axis: Always check if you’re looking at temperature or solubility.
- Misinterpreting values: Interpolate between points for accurate results.
- Not recognizing the difference between grams and moles: Pay attention to units.
As we've explored various problems and techniques for working with solubility curves, let's summarize what we've learned:
- Solubility curves illustrate how temperature affects solubility.
- Reading from these curves involves choosing the substance, finding the temperature, and determining solubility or vice versa.
- Understanding saturation and supersaturation concepts helps in interpreting solubility data.
- Key techniques like using a straight edge, checking scales, and understanding trends can improve accuracy and efficiency.
By mastering solubility curve problems, students not only excel in their chemistry coursework but also gain insight into practical applications like crystallization, pharmaceuticals, and environmental science.
What does it mean when a point on the solubility curve is above the line?

+
When a point is above the solubility curve, the solution is said to be supersaturated. This means that the solution contains more dissolved solute than what would normally dissolve at that temperature, making it unstable and prone to precipitate solute crystals.
Can you dissolve more solute at higher temperatures?

+
Generally, yes. For most substances, solubility increases with temperature. However, there are exceptions like sodium sulfate, whose solubility decreases with increasing temperature.
Why do some substances decrease in solubility with increasing temperature?

+
This can happen due to the endothermic or exothermic nature of dissolution. For endothermic processes, where dissolving the solute absorbs heat, an increase in temperature can shift the equilibrium towards dissolution. For exothermic processes, increasing temperature can favor the reverse reaction, decreasing solubility.
How can solubility curves help with environmental studies?

+
Solubility curves are essential in environmental science for predicting how pollutants dissolve in natural water bodies. Understanding how temperature changes affect solubility can help in managing contaminant release and water treatment processes.
What are practical applications of solubility curves?

+
Solubility curves have numerous practical applications in:
- Crystallization processes in chemical synthesis.
- Pharmaceutical manufacturing to optimize drug solubility.
- Food industry for designing specific textures and flavors.
- Environmental science to understand the mobility of pollutants in water.
- Geology for mineral dissolution and precipitation.