Skeleton Equations Worksheet: Master Balancing Equations Easily

Welcome to the exciting journey of mastering chemical equations through a focused worksheet on skeleton equations. This article will provide a step-by-step guide on how to balance equations in chemistry, which is fundamental for any aspiring chemist. From basic principles to advanced tips, we'll explore how understanding skeleton equations can significantly enhance your proficiency in chemistry.
Understanding Skeleton Equations
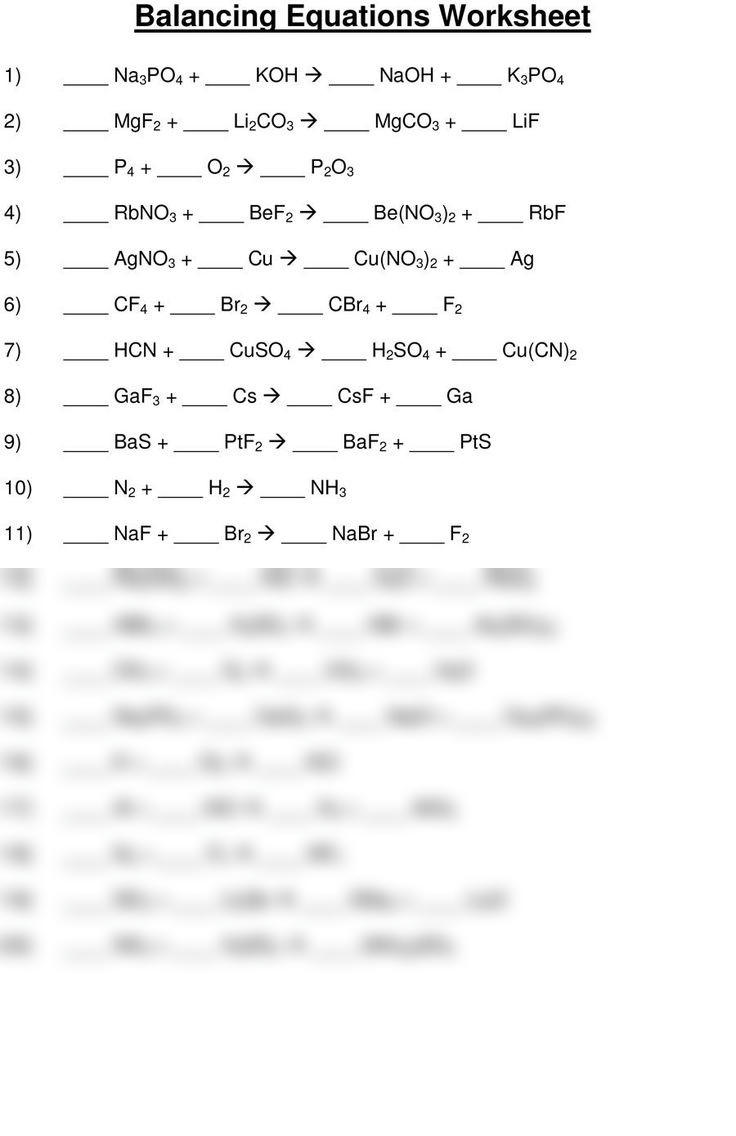
Before diving into the balancing of equations, it’s crucial to grasp what skeleton equations are:
- Definition: Skeleton equations are chemical equations written without balancing the numbers of atoms.
- Purpose: They show the reactants and products involved in a reaction but are not yet balanced, providing a starting point for students to practice balancing chemical equations.
An example of a skeleton equation might look like this: H₂ + O₂ → H₂O, which needs to be balanced to accurately represent the chemical reaction.
How to Balance Chemical Equations Using Skeleton Equations

Balancing chemical equations can seem daunting, but following these steps will make the process straightforward:
Step 1: Identify Reactants and Products

First, list all the reactants on the left side and products on the right side of the equation. For instance:
| Reactants | Products |
|---|---|
| H₂, O₂ | H₂O |

Step 2: Count the Atoms of Each Element
Count the number of atoms for each element on both sides. Here, there are 2 hydrogen atoms and 2 oxygen atoms on the left, and 2 hydrogen atoms with 1 oxygen atom on the right.
Step 3: Apply the Law of Conservation of Mass

Remember that the number of atoms of each element must be the same on both sides of the equation. This is where you start adjusting the coefficients:
- Place a 2 in front of H₂O to balance hydrogen: H₂ + O₂ → 2H₂O
- Then, adjust the O₂: H₂ + O₂ → 2H₂O becomes H₂ + 2O₂ → 2H₂O
Step 4: Balance One Element at a Time

When multiple elements need balancing, start with elements that appear in one reactant and one product. In our example, balance hydrogen first, then oxygen.
Step 5: Check and Adjust

Ensure all elements are balanced and the equation reflects the law of conservation of mass. Here, our final balanced equation is 2H₂ + O₂ → 2H₂O.
💡 Note: Always double-check your work. A common mistake is to forget to update all coefficients when one change is made, leading to an unbalanced equation.
Common Pitfalls and How to Avoid Them

Balancing equations is not without its challenges. Here are some common errors and strategies to avoid them:
- Improper Counting: Counting atoms incorrectly can lead to an unbalanced equation. Use a methodical approach to avoid this.
- Changing Subscripts: Never change the subscripts in the chemical formula as this changes the compound itself. Only adjust the coefficients.
- Forgetting Diatomic Elements: Elements like O₂, H₂, N₂, F₂, Cl₂, Br₂, and I₂ are diatomic and should be accounted for as such in the equation.
Advanced Tips for Balancing Complex Equations

When dealing with more complicated reactions, consider these advanced tactics:
- Use Fractions: If you encounter an uneven number, you can temporarily use fractions to balance the equation. Later, multiply everything by the least common multiple to clear fractions.
- Identify Reaction Patterns: Recognizing patterns like combustion, redox reactions, or precipitation can guide the balancing process.
Here is where your proficiency with skeleton equations pays off. By practicing with simpler reactions, you build the intuition needed to tackle more complex balancing scenarios.
Final Thoughts

In this exploration of balancing chemical equations, we’ve covered the basics of skeleton equations, detailed steps to balance them, common mistakes, and advanced techniques. Chemistry, like many sciences, is not just about memorizing facts but also about understanding principles and applying them logically. Your ability to balance chemical equations with ease is a testament to your growing understanding of the chemical world. Keep practicing, and soon, balancing even the most complex of equations will become second nature.
Why are skeleton equations important in learning chemistry?

+
Skeleton equations serve as a starting point for students to learn the mechanics of chemical reactions without the immediate pressure of balancing. They help in understanding the reactants and products involved, facilitating a deeper comprehension of how chemical changes occur.
Can you change the subscripts in a chemical formula to balance an equation?

+
No, you should never change the subscripts in a chemical formula. This action alters the identity of the substance. Instead, you adjust coefficients in front of the formulas to balance the equation.
How do you balance a complex chemical equation?

+
Balancing a complex equation involves several steps: Start by identifying the most complex compound or any element present in the least number of formulas. Use fractions if necessary, balance the key elements first, then work through the others, always ensuring the equation remains balanced for all elements.