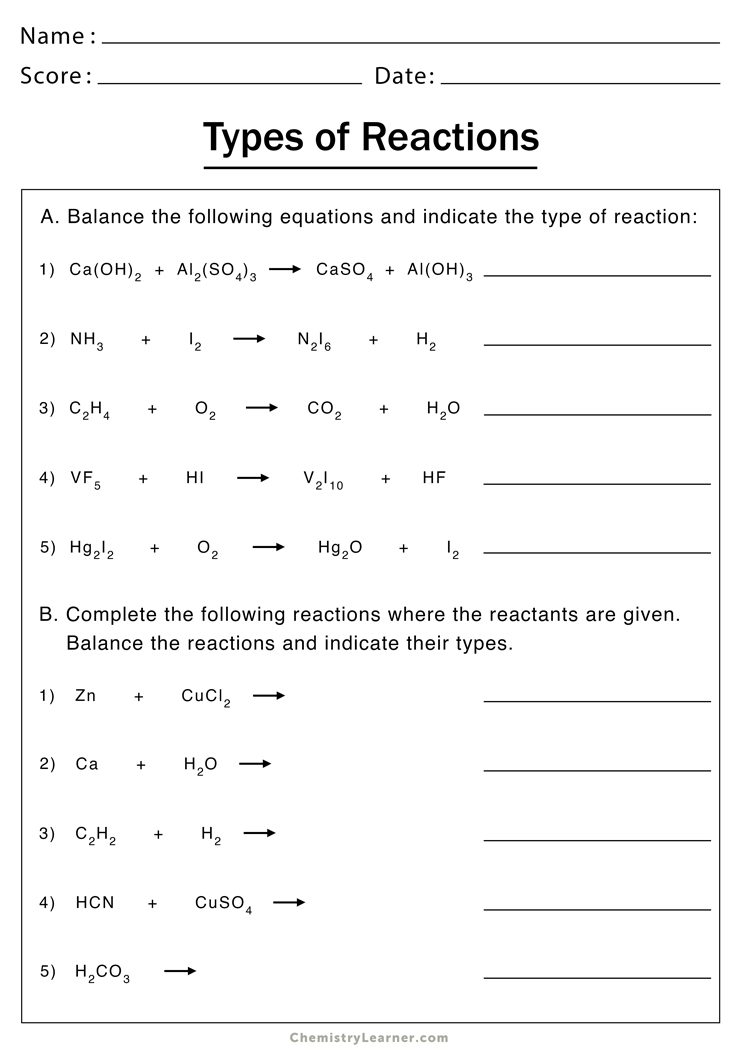
Single Replacement Reaction Worksheet: Complete Answers Guide

In the world of chemistry, reactions happen all around us, from the most mundane tasks like cooking or cleaning to the complex processes in industries. Understanding these reactions not only helps in demystifying the science behind everyday activities but also assists in tackling higher-level chemical education. One such fundamental reaction type students encounter is the single replacement reaction. If you're a student or an educator looking to dive deep into this topic, this comprehensive guide on a Single Replacement Reaction Worksheet will be your key to mastering this aspect of chemistry.
What is a Single Replacement Reaction?
A single replacement reaction, also known as a single displacement reaction, involves one element replacing another in a compound. This process occurs when a more reactive element displaces a less reactive one from its compound.
Here is the general formula for single replacement reactions:
A + BC → AC + B
Where:
- A is a single element.
- BC is a compound.
- AC is the new compound.
- B is the element displaced.
Identifying Single Replacement Reactions

To identify a single replacement reaction, look for these signs:
- A single element appears on one side of the equation.
- There is a clear displacement or replacement of one element for another in a compound.
- A change in color or the formation of bubbles often indicates that a reaction has occurred.
🔬 Note: Not all elements will participate in a single replacement reaction. The activity series of metals and non-metals helps determine if a reaction will occur.
Examples of Single Replacement Reactions

Here are some illustrative examples to help understand single replacement reactions:
- Metal Displacement: Zinc metal displacing copper from copper sulfate:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) - Non-Metal Displacement: Chlorine gas displacing bromide from potassium bromide:
Cl2(g) + 2KBr(aq) → 2KCl(aq) + Br2(l)
The Activity Series and its Importance

One critical aspect in determining if a single replacement reaction will occur is the activity series of metals and halogens. This series ranks elements by their relative reactivity:
- Metals like potassium, sodium, and lithium are at the top, being very reactive.
- Elements like gold and platinum are at the bottom, being less reactive.
Metal Activity Series:
| Element | Activity Level |
|---|---|
| Potassium (K) | Most reactive |
| Sodium (Na) | Very reactive |
| Magnesium (Mg) | Reactive |
| Aluminum (Al) | Moderately reactive |
| Iron (Fe) | Reactive |
| Hydrogen (H) | Ref point |
| Copper (Cu) | Less reactive |
| Silver (Ag) | Least reactive |
| Gold (Au) | Inert |

If the replacing metal is higher in the activity series, the reaction will take place. Similarly, for halogens, the displacement occurs when a more reactive halogen displaces a less reactive one.
Predicting Single Replacement Reactions

Predicting whether a single replacement reaction will occur can be simplified using the following steps:
- Identify the elements: Determine which elements are involved in the reaction.
- Consult the activity series: Check the reactivity of the elements involved.
- Check reactivity: If the element being added is more reactive than the one it’s supposed to displace, the reaction will likely occur.
Here’s an example:
Zn(s) + AgNO3(aq) → Zn(NO3)2(aq) + Ag(s)
In this case, Zinc (Zn) is higher on the activity series than Silver (Ag), so Zinc displaces Silver.
Practical Applications

Single replacement reactions have numerous practical applications, including:
- Galvanization: Coating iron with zinc to prevent rusting.
- Electroplating: Depositing a thin layer of one metal onto another for decoration or protection.
- Extracting Metals: Many metals are extracted from their ores through single replacement reactions, for example, the extraction of copper from copper oxide by heating with carbon (coke).
🔬 Note: Reactions in industries often require controlled conditions due to exothermic nature or the need for specialized equipment.
Common Misconceptions

Here are a few common misconceptions about single replacement reactions:
- Universal Replacement: Not all metals will displace any other metal. The reaction depends on the activity series.
- Hydrogen as a Metal: While hydrogen isn’t a metal, it’s often included in the activity series as a reference point since metals can replace hydrogen in acids or water.
- No Reaction with Less Reactive Elements: Reactions won’t occur if the displacing element is less reactive than the element it’s trying to replace.
⚗️ Note: Remember, understanding these reactions is not just about memorization but understanding the underlying principles of reactivity and electron transfer.
What makes a single replacement reaction occur?

+
A single replacement reaction occurs when a more reactive element displaces a less reactive element from its compound. The reaction is driven by the difference in reactivity, which can be determined using the activity series.
Why are some single replacement reactions not spontaneous?

+
If the element attempting to displace is less reactive than the element it's replacing, the reaction will not occur spontaneously because it would require additional energy to overcome the higher energy barrier of the less reactive element.
How does the activity series affect industrial processes?

+
The activity series is crucial in industrial applications, particularly in metal extraction where the energy efficiency and feasibility of reactions depend on the relative positions of elements in the series.
In summary, understanding single replacement reactions is fundamental to mastering chemistry. This guide has walked through the basics of identifying these reactions, their practical applications, common misconceptions, and offered a thorough breakdown of how to predict them using the activity series. Whether you’re a student grappling with homework or an educator preparing to teach, these insights will illuminate the path to chemical proficiency. Remember, the beauty of chemistry lies in its systematic nature, where understanding the rules of reactivity and electron behavior opens doors to numerous industrial and scientific advancements.