Simple Binary Ionic Compounds Worksheet: Easy Learning Guide

In the vast and intricate world of chemistry, understanding the basics forms the foundation for more complex learning. One such foundational concept is that of binary ionic compounds. These are compounds composed of just two elements, one being a metal that donates electrons (cation) and the other a non-metal that accepts electrons (anion). This process of electron exchange results in an electrostatic attraction known as an ionic bond. If you're seeking a straightforward approach to master this topic, our Simple Binary Ionic Compounds Worksheet is here to guide you through this essential chemical bonding with clarity and ease.
Why Understanding Ionic Compounds is Essential

Ionic compounds play a pivotal role in several fields:
- Chemical Reactions: Predicting the products of reactions often involves understanding ionic compounds.
- Material Science: Many useful materials, from ceramics to electrolytes, are based on ionic bonds.
- Biology: Ionic compounds are crucial for life processes, like the function of salt bridges in proteins.
Overview of the Worksheet

Our worksheet is designed to break down the process of working with binary ionic compounds into digestible steps:
- Identify Cations and Anions: How to distinguish between metals that lose electrons and non-metals that gain electrons.
- Formula Writing: Learn the step-by-step process of writing chemical formulas.
- Naming Compounds: Understand the nomenclature rules for binary ionic compounds.
- Interactive Practice: Engage in exercises that reinforce understanding through repetition.
This comprehensive guide helps you build proficiency in a topic that can seem daunting at first but is actually quite logical once the patterns are understood.
Learning Objectives
By the end of this worksheet, learners should be able to:
- Distinguish between cations and anions within binary ionic compounds.
- Correctly write chemical formulas for binary ionic compounds based on the periodic table.
- Name binary ionic compounds by following standard chemical nomenclature rules.
- Apply the concepts learned to solve ionic compound-related problems and identify them in the real world.
Identifying Cations and Anions

The periodic table is your best tool for quickly determining which elements will form cations and anions:
- Cations: Typically, metals on the left side of the periodic table (alkali, alkaline earth, and transition metals) form positive ions. For example, Sodium (Na) loses one electron to become Na+.
- Anions: Non-metals on the right side, particularly from groups 15-17, form negative ions. Chlorine (Cl), for instance, gains one electron to become Cl-.
To remember this, think of metal ions as “metal cations,” hence “metals give (cations)” and non-metals accept, forming anions.
💡 Note: Some elements, like hydrogen, can act as both cations and anions depending on the context; however, for binary ionic compounds, you'll mainly deal with the above patterns.
Writing Chemical Formulas
Here’s how to write the formula of a binary ionic compound:
- List the ions: Write the metal ion (cation) first, followed by the non-metal ion (anion).
- Balance Charges: The total positive charge must equal the total negative charge. To achieve this:
- Use the absolute value of the anion's charge as the subscript of the cation, and vice versa.
- Reduce to the lowest whole number ratio if possible.
- Formulate: Combine these subscripts to form the formula.
For example, to form Aluminum Oxide:
- Aluminum (Al) loses three electrons to become Al3+.
- Oxygen (O) gains two electrons to become O2-.
The formula balances when we use the charges to determine subscripts:
| Cation | Anion | Subscripts | Balanced Formula |
|---|---|---|---|
| Al3+ | O2- | 2(Al), 3(O) | Al2O3 |

📌 Note: When reducing to the lowest ratio, remember to write the formula with the smallest whole numbers possible.
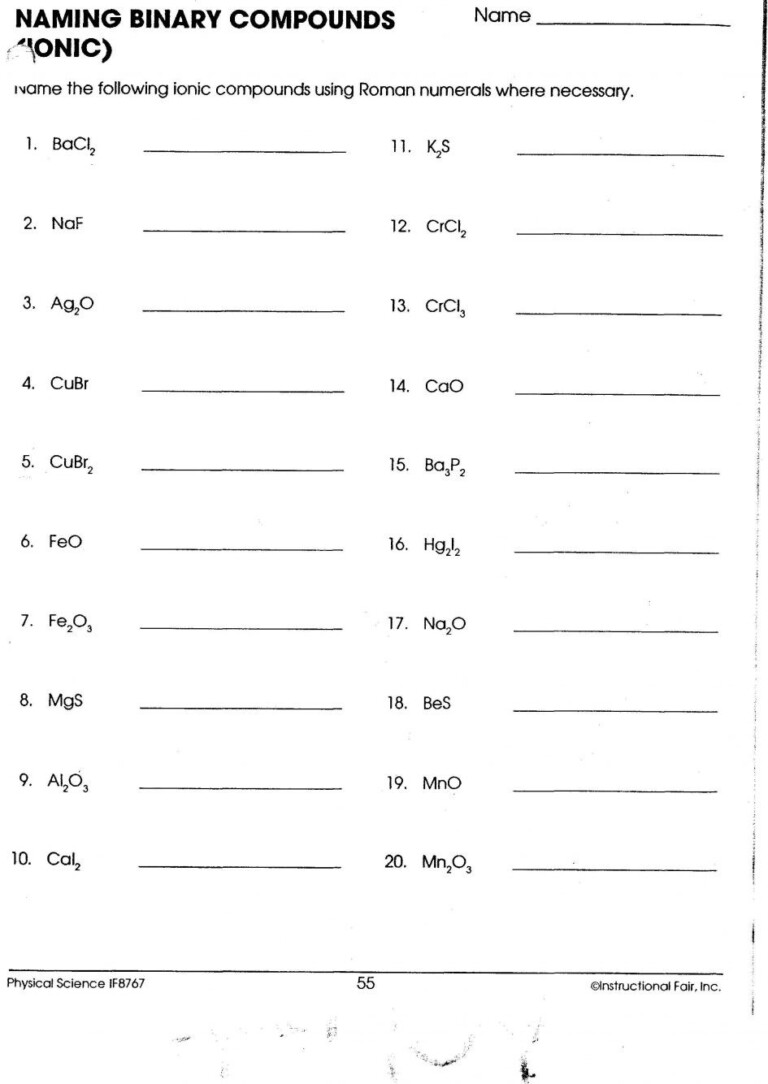
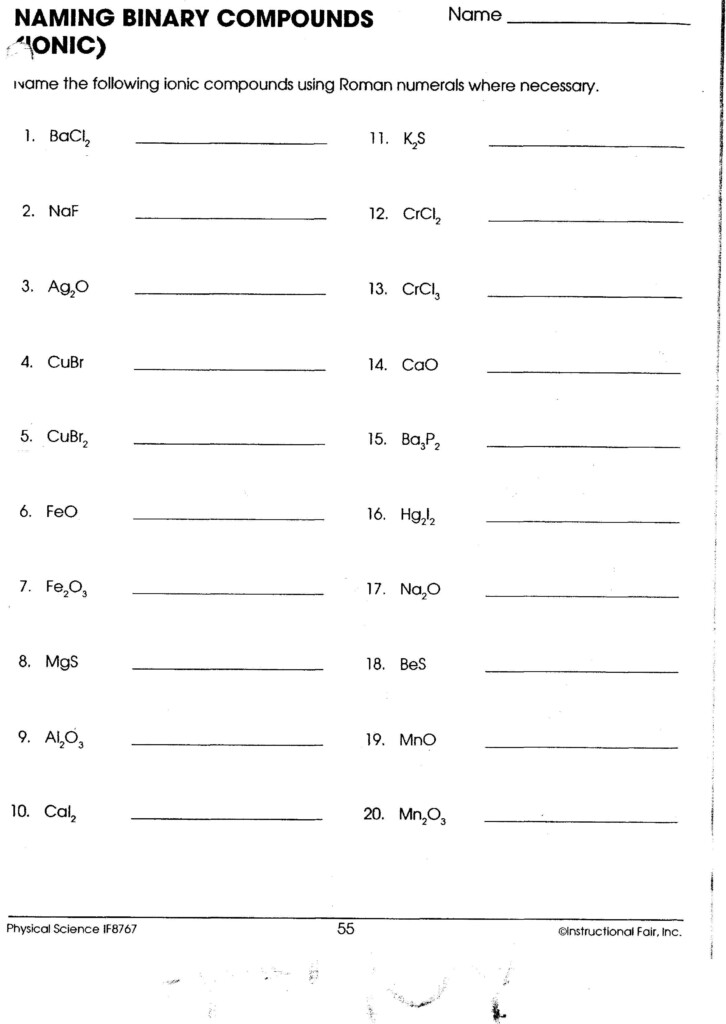
Naming Binary Ionic Compounds

Naming these compounds follows a systematic method:
- Write the name of the metal (cation) first, as it appears in the periodic table.
- Write the name of the non-metal, but change its ending to "-ide"
- For metals with multiple charges (like Iron), use Roman numerals to indicate the charge.
Take our previous example of Aluminum Oxide:
- Al (aluminum) + Ox (oxide) = Aluminum Oxide.
For transition metals:
- Fe (Iron) with a charge of +2 in Iron(II) Oxide or +3 in Iron(III) Oxide.
💡 Note: Remember to use Roman numerals for transition metals with varying charges to avoid ambiguity.
Practice with Interactive Exercises

Our worksheet includes exercises to cement the concepts you’ve learned:
- Multiple Choice Questions: Test your understanding of key concepts like ion charges.
- Fill in the Blanks: Practice naming and writing formulas for given compounds.
- Short Answer: Explain concepts in your own words to reinforce learning.
Here’s an example of what you might encounter:
Question: Write the chemical formula for a compound with a magnesium cation and a fluoride anion.
📌 Note: Remember, practice is crucial as it cements theoretical knowledge into applied skill.
In summary, mastering the understanding of binary ionic compounds opens doors to various areas of science and everyday life. This worksheet simplifies the process by providing clear instructions, practical examples, and interactive exercises that enhance your knowledge of chemical formulas and nomenclature. The concepts you’ve learned here are not only vital for academic pursuits but also for understanding the molecular structure of compounds we encounter daily. Through consistent practice and application, these foundational principles become second nature, allowing for a more profound exploration into the world of chemistry.
What is the difference between an ionic and a covalent bond?

+
Ionic bonds form through the transfer of electrons, creating oppositely charged ions that are attracted to one another. Covalent bonds, however, occur when atoms share electrons, forming stable molecules. The difference mainly lies in how electrons are managed between the atoms.
Why are Roman numerals used in naming some ionic compounds?

+
Roman numerals are used for metals with multiple possible charges (valencies) to indicate the specific charge the metal has in that compound. This helps to avoid confusion and precisely identify the compound, especially for transition metals.
Can a metal form an anion?

+
While metals generally form cations by losing electrons, under certain conditions, some can form anions or complex anions. For example, mercury can form Hg2- in some compounds. However, this is rare and usually involves higher oxidation states or special compounds.