Mastering Significant Figures and Scientific Notation: 5 Key Answers
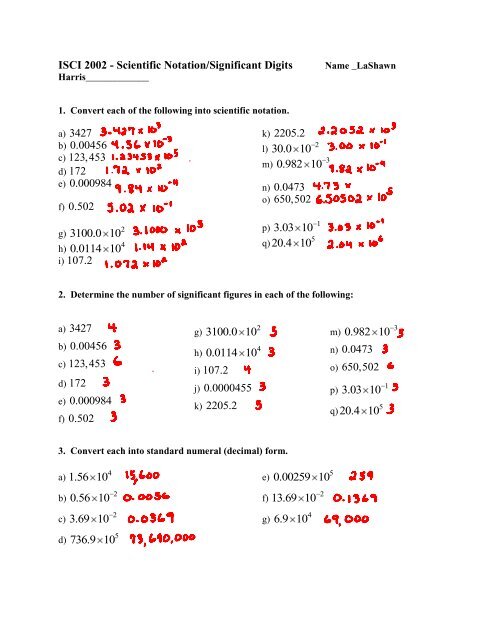
⚠️ Note: This tutorial is in HTML format and contains interactive elements. Ensure to view it in an HTML parser or web browser for full interactivity.
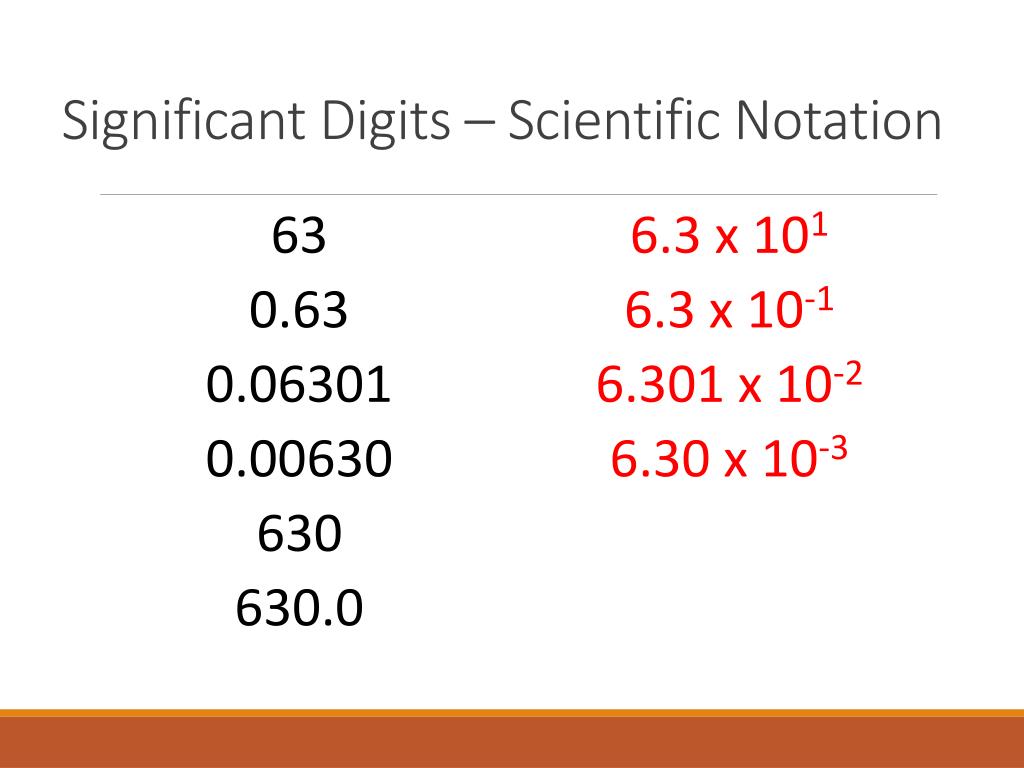
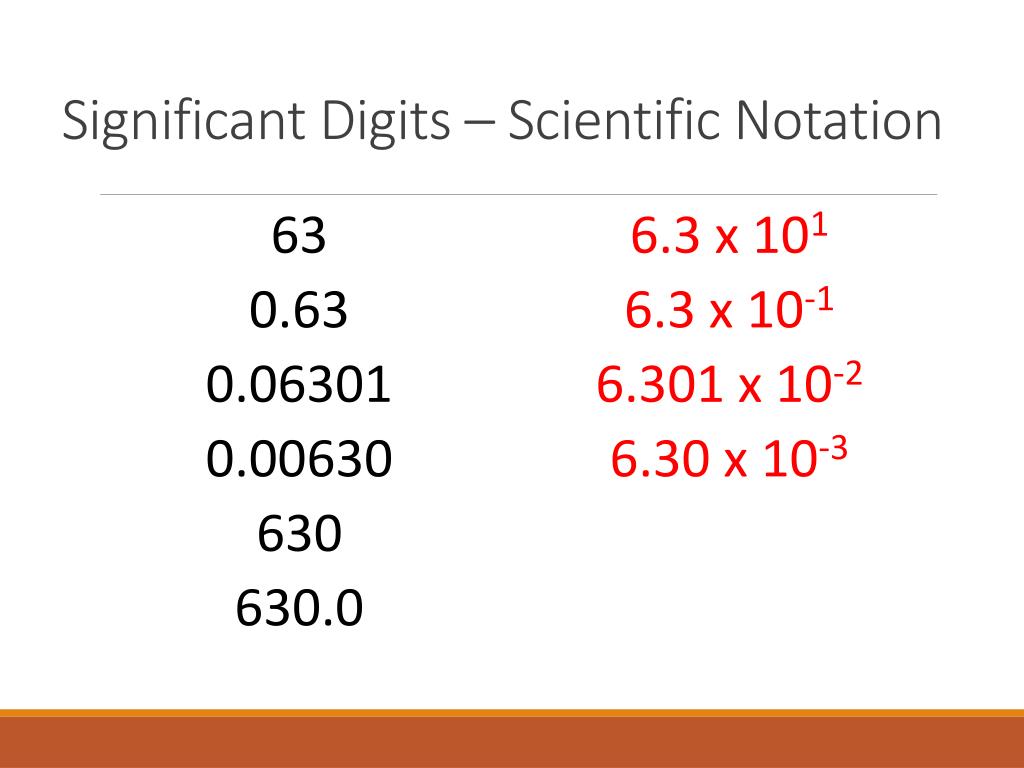
Understanding Significant Figures

Significant figures, also known as sig figs, are the digits in a number that carry meaning contributing to its precision. In scientific and engineering contexts, understanding how to identify and use significant figures is crucial for data accuracy. Here’s a breakdown of what they are:
- Non-zero digits: All digits from 1 to 9 are always significant.
- Leading zeros: Zeros at the beginning of a number, such as in 0.045, are not significant.
- Trailing zeros: Zeros at the end of a number with a decimal point are significant. For example, 20.00 has four significant figures.
- Zeros between non-zero digits: Zeros between two other digits are significant, like 405 has three significant figures.
Rules for Counting Significant Figures

- Non-zero digits: Count all of them.
- All zeros between non-zero digits: Count these as well.
- All trailing zeros in a number with a decimal point: Count these zeros.
- All digits in a number expressed in scientific notation: Count them all.
💡 Note: Remember, a zero between non-zero digits is always significant, but leading zeros never are unless they are after the decimal point.
Scientific Notation for Convenience

Scientific notation is used to express very large or very small numbers succinctly. It involves writing a number as a product of two factors: a coefficient that’s between 1 and 10, and a power of 10.
Converting to Scientific Notation

To convert a number to scientific notation:
- Move the decimal point so that the number is between 1 and 10 (the coefficient).
- Count the number of places the decimal point has been moved (the exponent).
- If you moved the decimal point to the left, the exponent is positive. If to the right, it’s negative.
Here's an example:
2567849.33 → 2.56784933 × 10^6
Performing Calculations with Significant Figures

When performing mathematical operations, the number of significant figures in the result depends on the numbers you started with:
- Addition and Subtraction: The result should be rounded to the least number of decimal places of the numbers being added or subtracted.
- Multiplication and Division: The result should have the same number of significant figures as the number with the fewest significant figures.
Practical Examples
| Operation | Numbers | Result | Significant Figures |
|---|---|---|---|
| Addition | 2.4 + 1.50 | 3.9 | 1 |
| Subtraction | 3.2 - 1.95 | 1.25 (Rounded to 1.3) | 2 |
| Multiplication | 4.5 × 3.0 | 13.5 (Rounded to 14) | 2 |
| Division | 5.0 ÷ 2.5 | 2.0 | 2 |

Importance of Sig Figs in Science

In scientific research, precision and accuracy are paramount. Significant figures tell us:
- The precision of measurements: If an experiment measures a quantity to be 3.2, this number has two significant figures, implying a precision level of about ±0.05.
- The reliability of results: The number of significant figures in the answer gives a sense of the trustworthiness of the result.
- Guiding future experiments: Knowing the precision of initial data helps in designing experiments to achieve better accuracy.
Scientific Notation and Dimensional Analysis
Dimensional analysis, or the factor-label method, often relies on scientific notation to manage the scale of units. Here’s how:
- Consistency in units: Scientific notation allows easy conversion of units, making comparisons and calculations straightforward.
- Handling extreme values: When dealing with large or small numbers in different units, scientific notation provides a simple method to navigate these scales.
- Error estimation: By tracking significant figures, scientists can estimate errors in dimensional analysis calculations, ensuring accuracy.
In summary, understanding significant figures and scientific notation isn’t just about knowing the rules; it’s about appreciating their roles in improving accuracy, reducing error, and facilitating complex calculations. Both are integral tools for scientific inquiry and precise measurements in various fields, from physics to engineering to chemistry.
Why are trailing zeros in a whole number considered significant in scientific notation?

+
In scientific notation, trailing zeros in a whole number are considered significant because they indicate the precision of the measurement. For example, 50000 has five significant figures if written as 5.0000 × 10^4, conveying a different precision than simply writing 5 × 10^4.
How can you tell if a zero is significant?

+
A zero is significant if it’s between two non-zero digits, after a non-zero digit and a decimal point, or if it’s after a non-zero digit in a number expressed in scientific notation.
What’s the significance of significant figures in practical applications?

+
Significant figures are crucial for determining the precision of measurements, guiding the design of experiments for better accuracy, and ensuring that mathematical calculations do not introduce unnecessary or misleading precision.
What is the role of scientific notation in computer programming or data analysis?

+
Scientific notation is commonly used in computer programming and data analysis to handle very large or very small numbers efficiently, reducing errors due to float-point representation issues and simplifying data processing and output formatting.



