Saturated vs. Unsaturated Solutions: Ultimate Worksheet Guide

The world of chemistry is filled with intricacies, each concept building upon the last to help us understand the natural world around us. In this comprehensive guide, we will explore saturated and unsaturated solutions, offering clarity on these fundamental terms. We'll dive into what they mean, how they behave, and why this knowledge is crucial in various fields from science to industry. Here, we present you with the ultimate worksheet guide on saturated vs. unsaturated solutions.
The Basics of Solutions

Before delving into specifics, let’s first understand what a solution is. A solution comprises a solute (the substance being dissolved) and a solvent (the substance that dissolves the solute). Common examples include salt water (sodium chloride as the solute, water as the solvent) and sugar water.
What is a Solution?

- Solute: The component dissolved in the solvent.
- Solvent: The component in which the solute dissolves.
- Solubility: The maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature.
Saturated Solutions
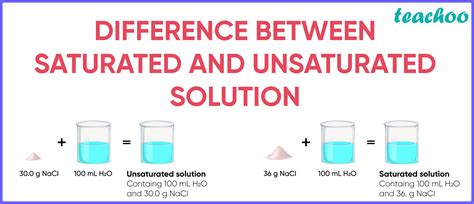

What is a Saturated Solution?

A solution reaches saturation when no more solute can dissolve in the solvent at a given temperature and pressure. At this point:
- The solvent has taken up the maximum amount of solute it can hold.
- If more solute is added, it will not dissolve; instead, it will remain as a solid or precipitate.
🌡️ Note: Temperature affects solubility; an increase typically allows more solute to dissolve, but cooling may cause the excess to crystallize out.
How to Create a Saturated Solution

- Choose your solute and solvent.
- Add solute to the solvent until it can’t dissolve anymore.
- Stir the mixture thoroughly to ensure saturation.
It’s important to be aware that:
- The solution must be at equilibrium; this means the rate of dissolving equals the rate of precipitation.
- Adding heat can temporarily push the solution past saturation, creating a supersaturated solution, where solute comes out of solution upon cooling.
Unsaturated Solutions


What is an Unsaturated Solution?

An unsaturated solution has less solute dissolved in the solvent than it has the capacity to dissolve. Key characteristics include:
- The solvent can dissolve more solute.
- If more solute is added, it will dissolve.
How to Identify an Unsaturated Solution

You can identify an unsaturated solution by:
- Adding more solute and observing if it dissolves.
- Checking against the solubility curve for that particular solute-solvent system.
Knowing whether a solution is unsaturated helps in:
- Determining how much more solute can be added to reach saturation.
- Assessing the stability and concentration of a solution for specific applications.
Application and Importance

The distinction between saturated and unsaturated solutions is not merely academic; it has practical implications:
| Field | Relevance |
|---|---|
| Chemistry | Understanding reaction rates, solubility product, and precipitation. |
| Pharmaceuticals | Ensuring drug solubility and bioavailability. |
| Environment | Analyzing water quality and pollutant behavior. |

This knowledge aids in:
- Manufacturing processes where solution concentration is critical.
- Formulating products like cleaning agents, fertilizers, and cosmetics.
- Environmental analysis and control of pollutants in water.
Worksheet Guide for Students

To help students master these concepts, here is an extensive worksheet guide:
Saturated vs. Unsaturated Solutions

Section 1: Understanding Concepts
- Define a saturated solution in your own words.
- Describe how temperature affects solubility.
- List three ways to identify an unsaturated solution.
Section 2: Application Problems
Example problem:
- If you dissolve 36 grams of sugar in 100 grams of water at 25°C, will the solution be saturated or unsaturated? (Given: Solubility of sugar at 25°C in water is 203.9 g/100 g H2O)
Section 3: Interactive Experiment
- Mix salt and water at room temperature. Describe what happens.
- Add more salt until no more dissolves. What does this indicate?
- Now heat the mixture slightly. Does the solubility change? Explain why.
⚗️ Note: Always perform experiments under adult supervision for safety.
In this thorough exploration of saturated and unsaturated solutions, we've covered the fundamental principles, practical applications, and provided students with a guide to further their learning. Understanding these concepts not only prepares students for higher-level chemistry but also provides a foundation for understanding real-world phenomena where solutions play a crucial role. Remember, whether you're making lemonade or analyzing environmental samples, knowing when your solution is saturated or unsaturated can make all the difference.
What happens if you try to dissolve more solute in a saturated solution?
+
If you attempt to add more solute to a saturated solution, it will not dissolve and instead remain as a solid or precipitate at the bottom of the solution.
Can temperature change the saturation point of a solution?

+
Yes, temperature can significantly affect the saturation point. Typically, increasing the temperature allows more solute to dissolve, thus altering the saturation point.
How can you tell if a solution is unsaturated?

+
A solution is unsaturated if more solute can be dissolved in it. This can be tested by adding more solute to see if it dissolves or by comparing the current concentration to the solubility curve.
Why is understanding saturation important in industry?

+
In industry, understanding saturation is crucial for processes like chemical reactions, product formulation, and wastewater treatment, where solubility affects product quality, efficiency, and environmental impact.
Related Terms:
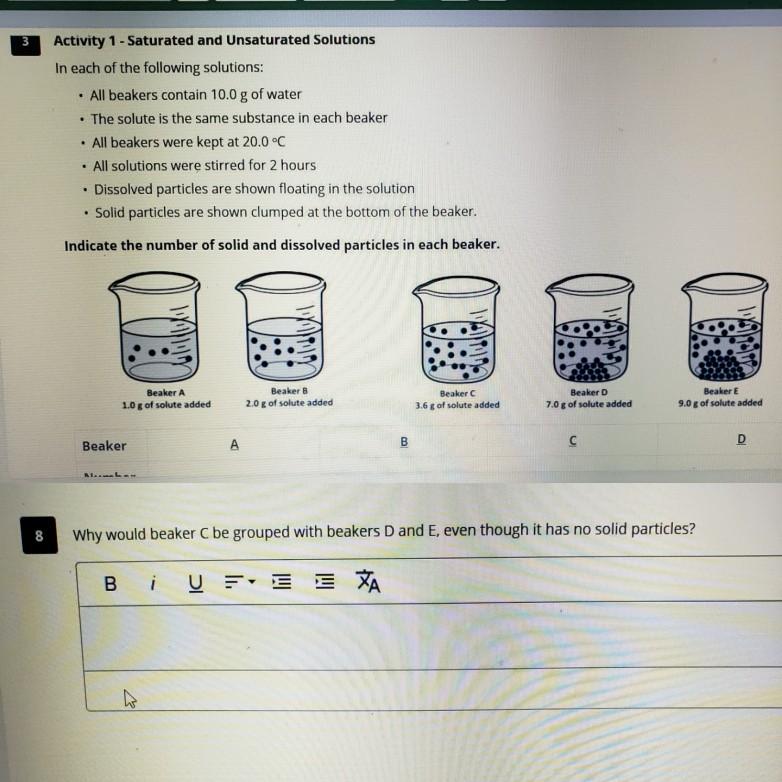
- Which beakers represent unsaturated solutions
- Saturated and unsaturated solutions ck12