5 Fun Steps for Red Cabbage pH Experiments

Red cabbage, known scientifically as Brassica oleracea, is not just a versatile vegetable for cooking but also an excellent tool for engaging in educational science experiments. Its rich anthocyanin content turns it into a natural pH indicator, making it perfect for kids and adults alike to explore the fascinating world of chemistry. Here are five fun steps to conduct red cabbage pH experiments that will both educate and entertain.
Step 1: Preparing the Red Cabbage Juice

The first step in our red cabbage pH experiment involves making the indicator solution:
- Chop one head of red cabbage into small pieces.
- Boil the chopped cabbage in about 500ml of water for about 15 minutes, until the water turns a deep purple.
- Allow it to cool slightly, then strain out the liquid into a heatproof container.
- Let the liquid cool completely; this is your pH indicator solution.
🔬 Note: If the color isn’t a vibrant purple, the indicator might not work effectively. Red cabbage should yield a rich color due to its high anthocyanin content.

Step 2: Collecting Household Substances

Now, gather an assortment of household items to test:
- Lemon juice (acidic)
- Vinegar (acidic)
- Baking soda solution (basic)
- Laundry detergent (basic)
- Water (neutral)
These substances cover a range of pH values, providing a spectrum of reactions when mixed with the cabbage juice.
Step 3: Setting Up the Experiment

With your indicator and test subjects at the ready, set up your experiment:
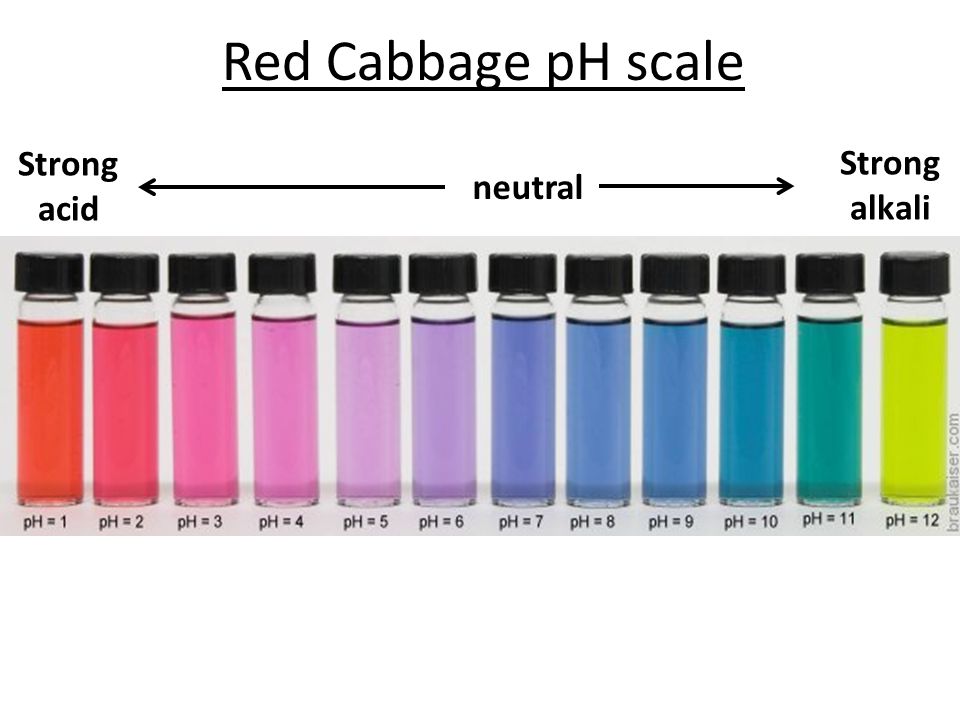
| Substance | Color Change Expected |
|---|---|
| Lemon Juice | Red/Pink |
| Vinegar | Pink |
| Baking Soda | Blue/Green |
| Laundry Detergent | Blue/Green |
| Water | Purple |

Place small cups or test tubes for each substance and label them accordingly. Add equal parts of the indicator to each container to observe the color changes.
Step 4: Conducting the Test

Add a few drops of your household items into the respective containers with the cabbage juice:
- Watch for immediate color changes.
- Record the results. Document how each substance affects the color of the cabbage juice.
🧪 Note: The accuracy of color changes can be enhanced by comparing results to a universal indicator chart for more precise pH readings.

Step 5: Analyzing Results and Making Observations

After conducting the tests, analyze the results:
- Discuss which substances turned the cabbage juice red, pink, or blue.
- Explain what the color changes signify in terms of pH - acids will turn the juice pink/red, and bases will turn it blue/green.
- Consider how the concentration of substances might influence results.
Your findings can be compared with pH charts or universal indicator papers for further validation.
📊 Note: This experiment not only teaches about pH but also about the behavior of natural indicators which can vary slightly in sensitivity.
Final Thoughts

Engaging in a red cabbage pH experiment provides a vivid, interactive way to learn about the basic principles of chemistry. By observing how substances alter the color of the cabbage juice, participants gain a practical understanding of pH levels, acids, bases, and how these interact with natural indicators. Moreover, the experiment fosters curiosity and promotes hands-on learning, making science accessible and enjoyable for everyone. Whether you’re a teacher, a parent, or just someone with a passion for science, this experiment is a colorful entry into understanding the world around us through simple kitchen ingredients.
What is pH?

+
pH stands for “potential of hydrogen,” which is a measure of the acidity or alkalinity of a solution. A pH below 7 is acidic, 7 is neutral, and above 7 is alkaline or basic.
Why does red cabbage work as a pH indicator?
+
Red cabbage contains anthocyanins, which are sensitive to changes in pH, causing the juice to change colors when mixed with acids or bases.
Can this experiment be done with other vegetables?

+
Yes, other vegetables like purple grape juice or hibiscus flowers can also serve as pH indicators due to their anthocyanin content.