5 Essential Reaction Rate Worksheets for Chemistry Enthusiasts

In the dynamic world of chemistry, understanding how quickly reactions occur is crucial. Reaction rates, the speed at which reactants are transformed into products, lie at the heart of many chemical processes, from industrial applications to natural phenomena. For students and enthusiasts eager to master this subject, reaction rate worksheets are invaluable tools. These worksheets not only reinforce learning but also enhance problem-solving skills, application, and understanding of kinetics. Here are five essential worksheets designed to cater to various learning levels and interests in chemistry:
1. Basic Introduction to Reaction Rates
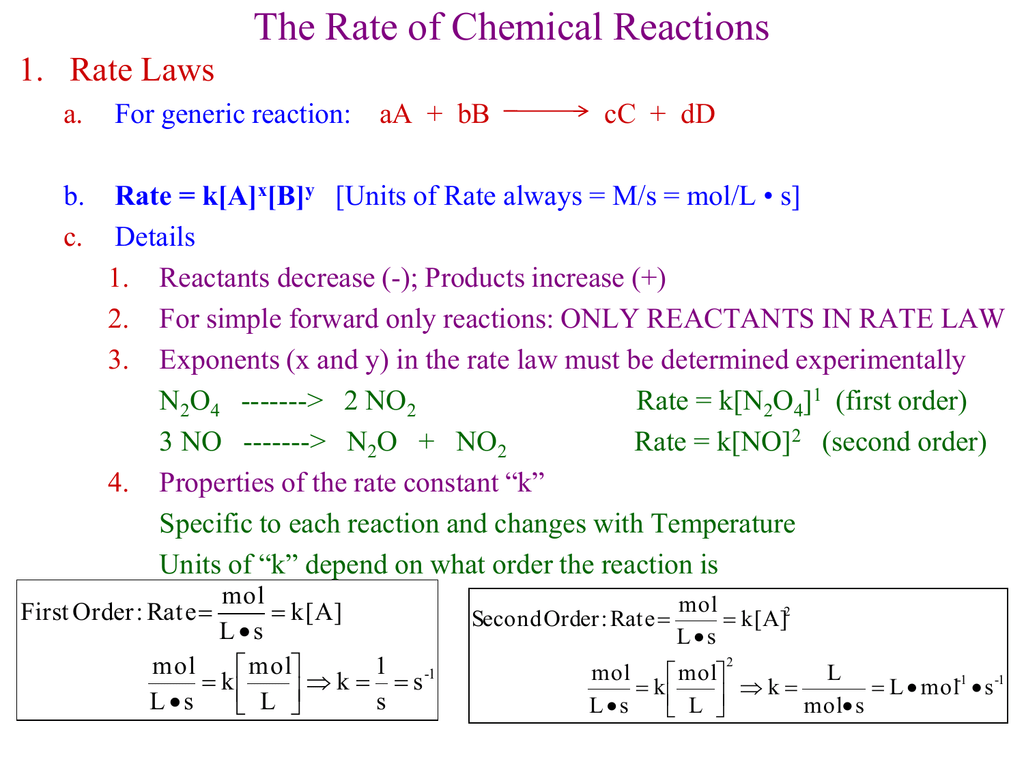

This worksheet serves as an introduction to reaction rates, suitable for high school students or beginners:
- Definition and Measurement: Understand how reaction rates are defined, measured in moles per liter per second, and why they are important.
- Factors Affecting Rates: Learn about temperature, concentration, catalysts, and surface area’s impact on reaction rates.
- Problem-Solving: Contains simple problems where students calculate rates from provided data.
🔬 Note: Ensure a clear grasp of units and conversion factors to avoid confusion in calculations.
2. Concentration-Time Curves and Initial Rates

Progressing from the basics, this worksheet focuses on:
- Graph Analysis: Interpret concentration-time graphs to determine initial rates and half-lives.
- Rate Law: Introduction to rate law expression and how initial rates can help derive them.
- Real-Life Applications: Examples from biological systems or chemical engineering where concentration-time relationships are key.
| Scenario | Concentration (mol/L) | Time (s) | Initial Rate (mol/L/s) |
|---|---|---|---|
| Reaction 1 | 0.02 | 5 | 0.004 |
| Reaction 2 | 0.01 | 10 | 0.001 |

3. Equilibrium and Reaction Rates

When reactions reach equilibrium, rates can seem confusing. This worksheet:
- Equilibrium Constants: Relate reaction rates to equilibrium constants (K).
- Graphical Interpretation: Plots to understand how the reaction rate changes at equilibrium.
- Catalysts: Explore how catalysts influence reaction rates without affecting equilibrium constants.
4. Advanced Kinetics: Activation Energy and the Arrhenius Equation

For those delving deeper into kinetics, this worksheet covers:
- Arrhenius Equation: Derive rate constants from temperature changes.
- Catalysis and Mechanisms: Detailed analysis of catalyst behavior and reaction mechanisms.
- Data Analysis: Graph and calculate activation energy from experimental data.

5. Industrial Applications of Reaction Rates

This worksheet explores the practical side of reaction rates:
- Case Studies: Analyze reactions in the petrochemical industry, pharmaceuticals, and food processing.
- Optimization: Learn strategies to optimize reaction conditions for industrial efficiency.
- Safety Protocols: Discuss the importance of understanding reaction rates in preventing accidents.
Engaging with these worksheets will not only build a foundational understanding of reaction rates but also encourage critical thinking and application of theoretical knowledge to real-world scenarios. The exercises, from basic calculations to complex problem-solving, provide a structured way for chemistry enthusiasts to deepen their understanding.
Why are reaction rates important in chemistry?
+
Reaction rates are essential because they dictate the speed at which chemical reactions occur. This knowledge is crucial for:
- Predicting outcomes: Knowing how fast a reaction will proceed helps predict the formation of products.
- Controlling processes: In industries, reaction rates inform process control, safety measures, and efficiency optimization.
- Understanding mechanisms: By studying rates, chemists can infer the pathways of chemical reactions, enhancing fundamental knowledge.
How can I improve my understanding of reaction rates?

+
Here are some tips to deepen your comprehension:
- Visual aids: Use concentration-time graphs and visualize the progress of reactions.
- Practice problems: Solve diverse reaction rate problems to get familiar with different scenarios.
- Understand units: Clear understanding of units and conversion factors is crucial for correct calculations.
- Research: Delve into case studies or real-world applications of reaction rates for practical insight.
What role do catalysts play in reaction rates?

+
Catalysts accelerate the rate of chemical reactions without being consumed in the process. They do this by:
- Lowering activation energy: By providing an alternative reaction pathway with a lower energy barrier.
- Increasing frequency of effective collisions: Catalysts can orient reactants or increase their surface area for reactions.
- Not affecting equilibrium: Catalysts only change the rate, not the equilibrium position of a reaction.