Mastering Quantum Numbers: Engaging Practice Worksheets

In the fascinating realm of atomic physics, understanding quantum numbers is crucial. They provide us with the coordinates necessary to describe the position and energy levels of electrons within an atom. Engaging with quantum numbers can seem daunting at first, but with the right practice worksheets, it can become a captivating learning experience. This post delves into the essentials of quantum numbers, offering practical exercises through worksheets designed to enhance comprehension and retention.
Understanding Quantum Numbers

Quantum numbers play a pivotal role in defining the state of electrons in an atom. Here’s an overview of what each quantum number signifies:
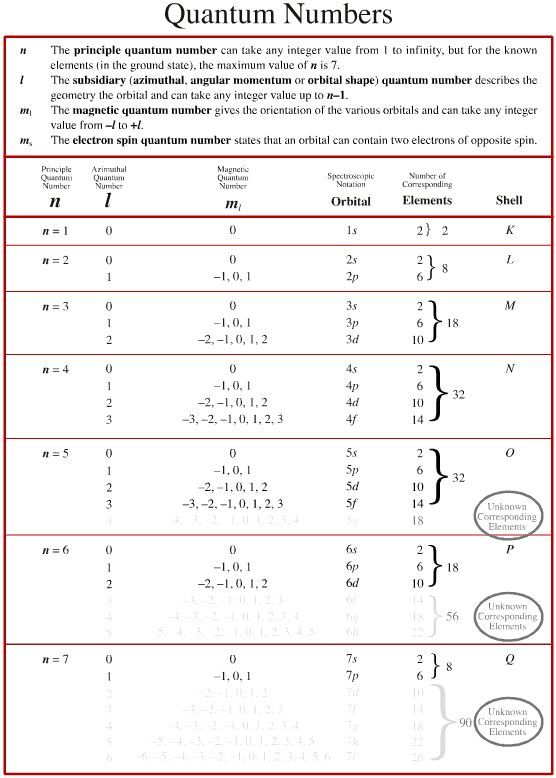
- Principal Quantum Number (n): Indicates the energy level or shell of an electron, where higher numbers indicate larger, higher-energy orbitals.
- Angular Momentum Quantum Number (l): Describes the shape of the atomic orbital. The values range from 0 to (n-1) and are given the symbols s, p, d, f, etc., for l=0,1,2,3, respectively.
- Magnetic Quantum Number (ml): Determines the orientation of the orbital in space. Its values range from -l to +l, giving the number of orbitals for each ‘l’ value.
- Spin Quantum Number (ms): Denotes the spin orientation of the electron, either +½ or -½, indicating clockwise or counterclockwise spin.
Designing Quantum Numbers Worksheets
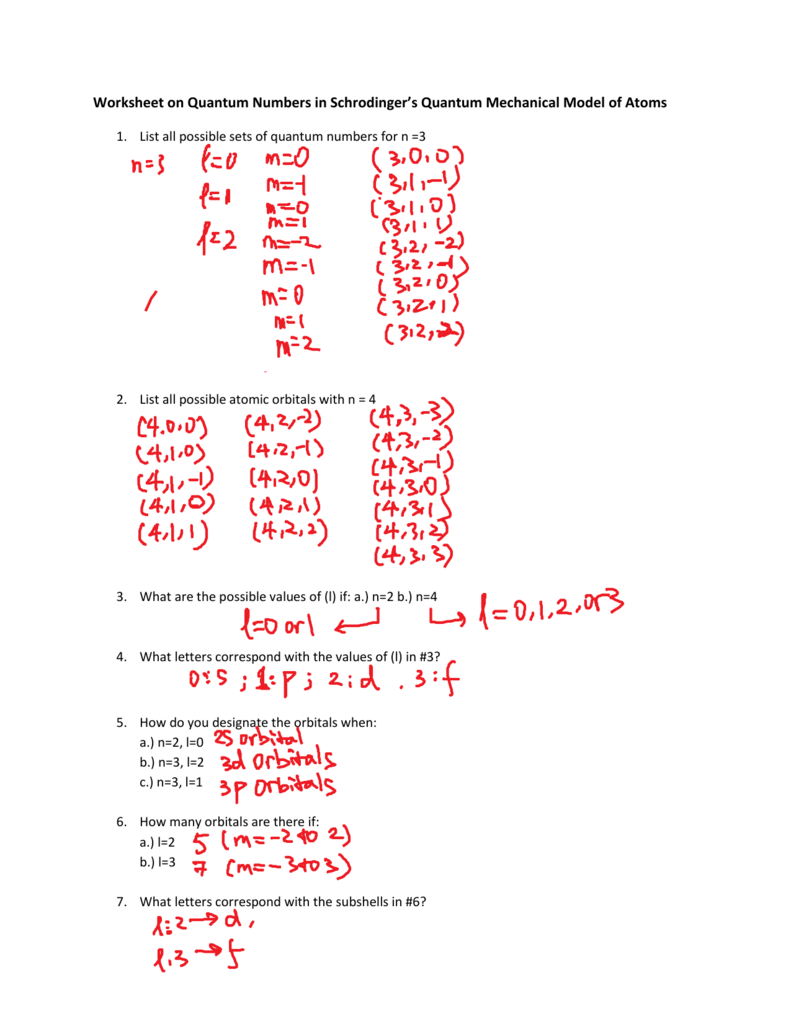
Creating effective practice worksheets for quantum numbers involves several steps:
- Identify Key Concepts: Begin by focusing on the principal, angular, magnetic, and spin quantum numbers. Each worksheet should introduce these concepts progressively.
- Practical Application: Include exercises where students have to fill in the values of quantum numbers for different electrons in atoms. For example:
Element Orbital n l ml ms Hydrogen 1s [ ] [ ] [ ] [ ] 
- Conceptual Understanding: Pose questions that require understanding, such as, “Explain why the ’s’ orbital has only one spatial orientation.”
- Visuals and Diagrams: Use diagrams to illustrate electron configurations and orbital shapes to help students visualize quantum number concepts.
- Interactive Challenges: Implement puzzles or ‘match the quantum number’ games where students link given values to possible orbital configurations.
💡 Note: Ensure your worksheets reflect real-life applications to make learning more engaging. For instance, show how quantum numbers are used in spectroscopy or magnetic resonance imaging (MRI).
Worksheet Examples

Here are some examples of the types of questions and exercises you might include in your quantum numbers worksheets:
Basic Quantum Number Matching

- Match the symbol with its corresponding quantum number.
- Which quantum number determines the size and energy of the orbital?
Application Exercises

- Given the quantum numbers n = 2, l = 1, ml = -1, and ms = +1⁄2, what is the full electron configuration of this electron?
- Draw the possible electron configurations for an atom with principal quantum number 3.
Conceptual Questions

- How do the principal and angular momentum quantum numbers relate to the energy levels of electrons?
- What are the limitations of the Pauli exclusion principle in terms of quantum numbers?
Making Learning Memorable

To turn worksheets into a memorable learning tool, consider:
- Using color-coded diagrams to represent different quantum numbers.
- Incorporating historical anecdotes about the discovery of quantum mechanics.
- Providing real-world examples where understanding quantum numbers is crucial, like in quantum computing or the development of new materials.
- Encouraging students to create their own mnemonics or memory aids for remembering the roles of each quantum number.
By integrating these elements into your worksheets, you can turn abstract concepts into tangible learning moments.
💡 Note: Always review and adjust the difficulty of your worksheets based on student feedback to ensure they are challenging but achievable.
Wrapping up, mastering quantum numbers through practice worksheets not only solidifies fundamental knowledge of atomic physics but also enhances problem-solving skills. With thoughtful design and progressive complexity, these worksheets can transform an intimidating subject into an exciting exploration of the micro-world.
Why are quantum numbers important in chemistry?
+
Quantum numbers are crucial because they define the state of an electron in an atom, influencing its energy, location, and behavior, which in turn affects the atom’s chemical properties and reactivity.
How many values can the principal quantum number take?

+
The principal quantum number (n) can take any positive integer value from 1 to infinity, although in practice, we usually work with lower values.
Can the magnetic quantum number (ml) be zero?

+
Yes, ml can be zero when l is also zero, corresponding to the s orbitals where there is no spatial orientation.