Master Protons, Neutrons, Electrons with Our Practice Worksheet
Understanding the basic building blocks of matter—protons, neutrons, and electrons—is fundamental to any study in chemistry, physics, or related fields. These subatomic particles are not only essential for understanding atomic theory but also play crucial roles in various natural phenomena. This blog post aims to guide you through the intricacies of protons, neutrons, and electrons, offering insights through a detailed practice worksheet to help you master these concepts.
The Basics of Atomic Structure

At the heart of every atom lies the nucleus, surrounded by a cloud of electrons. Here’s a simple breakdown of these particles:
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles also residing in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels.
Charge, Mass, and Location

To truly grasp how these particles interact, let’s delve into their properties:
| Particle | Charge | Mass (u) | Location |
|---|---|---|---|
| Protons | +1 | 1.0073 | Nucleus |
| Neutrons | 0 | 1.0087 | Nucleus |
| Electrons | -1 | 0.0005486 | Orbitals around the nucleus |

⚠️ Note: The mass units used are atomic mass units (u), where 1 u ≈ 1.66 × 10-27 kg.
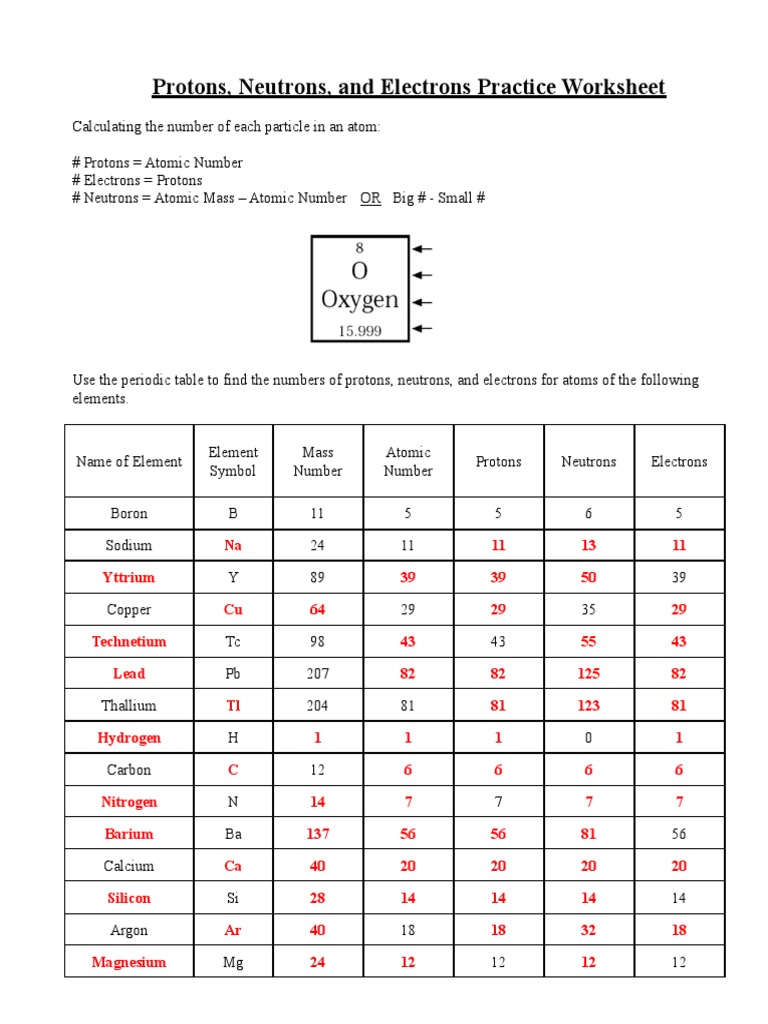
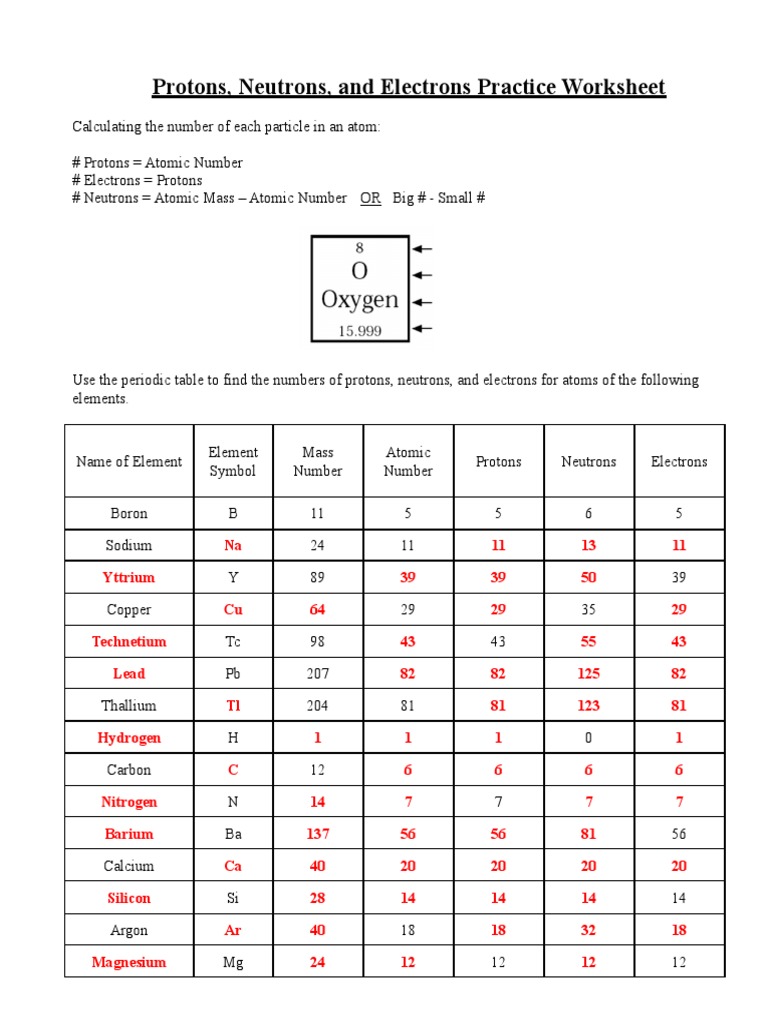
Practice Worksheet: Protons, Neutrons, and Electrons
Below, we present a series of practice problems to enhance your understanding of these fundamental particles:
Problem 1: Counting Protons, Neutrons, and Electrons

Given an element’s atomic number (Z) and its mass number (A), determine the number of protons, neutrons, and electrons:
- Example: For 35Cl, atomic number is 17, mass number is 35.
- Number of protons: 17
- Number of neutrons: A - Z = 35 - 17 = 18
- Number of electrons (in a neutral atom): equals the number of protons, so 17.
⚠️ Note: In a neutral atom, the number of electrons equals the number of protons. In ions, this might differ due to charge.
Problem 2: Isotope Identification

Identify the isotopes and their abundance in the following example:
- Element: Carbon ©
- Two isotopes: 12C and 14C
- 12C has 6 protons, 6 neutrons, and 6 electrons (neutral)
- 14C has 6 protons, 8 neutrons, and 6 electrons (neutral)
⚠️ Note: Isotopes of an element differ only in the number of neutrons.
Problem 3: Ion Formation

Understand how ions form:
- Example: Sodium (Na) losing one electron:
- Protons: 11
- Neutrons: 12
- Electrons (before): 11
- Electrons (after forming Na+): 10
To sum up, mastering protons, neutrons, and electrons allows you to comprehend atomic behavior, which is vital for understanding chemistry and physics. Through practical problems, we've explored how these particles contribute to the atom's identity, its charge, and mass. This foundation sets the stage for more advanced topics in atomic structure, chemical reactions, and the periodic table. Engaging with these exercises not only reinforces your learning but also prepares you for deeper explorations into the molecular world.
Why are the charges of protons and electrons important?

+
The charges are crucial for the atom’s stability and reactivity. Protons have a positive charge, while electrons have a negative charge, and these charges balance each other in a neutral atom.
What happens to the atomic mass if the number of neutrons changes?

+
When the number of neutrons changes, it forms isotopes with different atomic masses but the same atomic number. This affects the mass of the isotope but not its chemical behavior.
Can an atom lose or gain protons?

+
An atom does not typically lose or gain protons because doing so would change the element’s identity. However, nuclear reactions can transform elements by changing the number of protons in the nucleus.