5 Essential Properties of Matter: A Worksheet Guide

Understanding Matter: An Introduction to Its Essential Properties
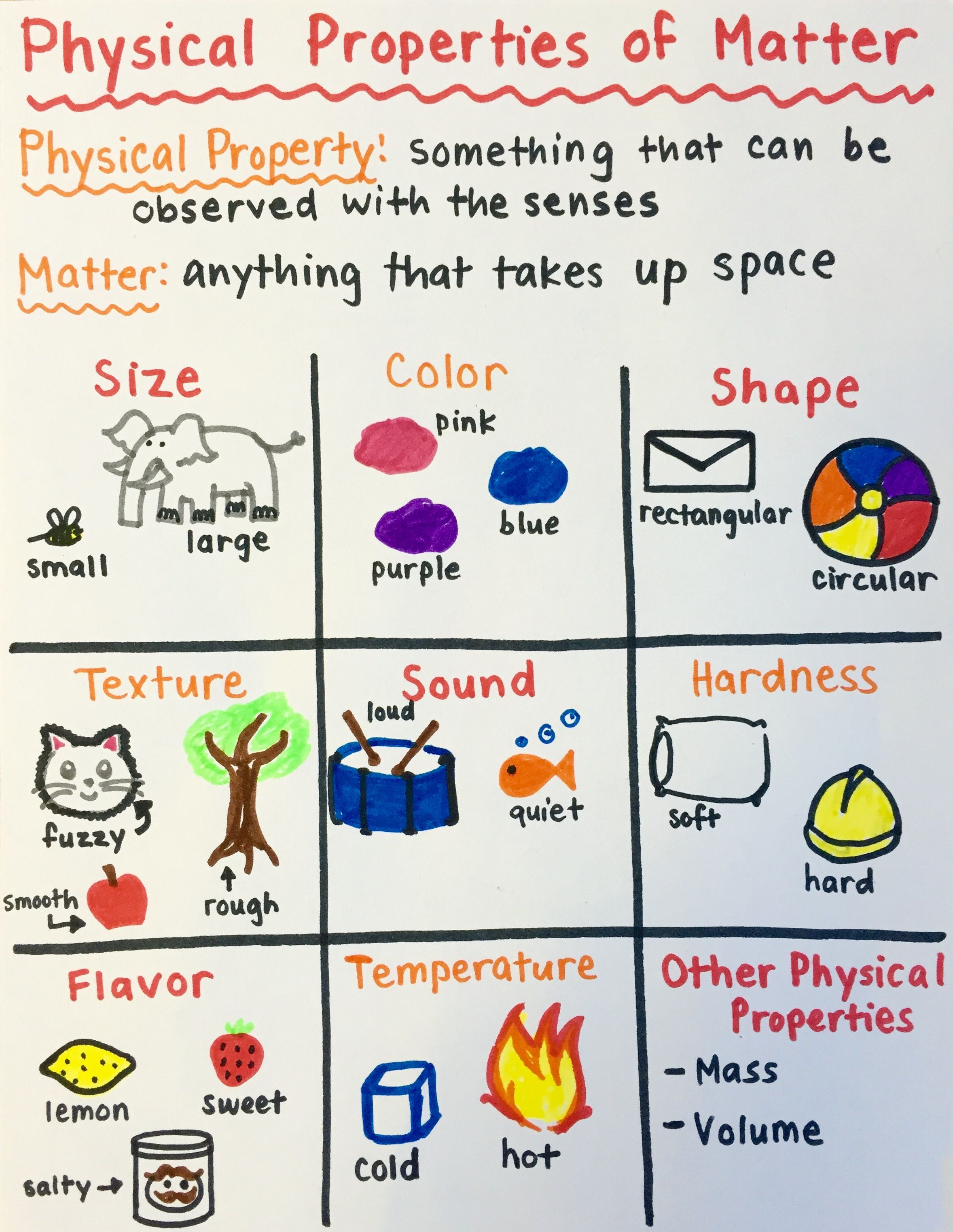
Matter, defined by its ability to occupy space and possess mass, forms the foundational substance of our physical universe. Understanding its properties is not just academic; it’s essential for practical applications in science, engineering, technology, and everyday life. This post will guide you through the five essential properties of matter that are crucial for anyone exploring chemistry, physics, or material science.

Mass: The Measure of Inertia

Mass refers to the amount of matter in an object, providing a measure of its inertia – the resistance an object has to changes in its state of motion. Here’s why mass is essential:
- Gravitational Attraction: Mass directly influences the gravitational pull an object experiences, affecting its weight when placed on different planets or moons.
- Inertia: It determines how an object will behave under forces; more mass means more resistance to acceleration or deceleration.
- Conservation of Mass: In chemical reactions, mass is neither created nor destroyed, making it a fundamental law in chemistry.
Volume: Space Occupation

The volume of a substance is the measure of the space it occupies. Volume can be:
- Calculated: Using formulas for regular shapes like cubes or cylinders.
- Displaced: Volume can be measured indirectly by the displacement of water when irregular objects are submerged.
- Changes: Volume can change with temperature or pressure, especially in gases.
👨🔬 Note: At absolute zero, the volume of an ideal gas would theoretically approach zero, but real gases don’t condense to zero volume because of intermolecular forces.
Density: A Connection Between Mass and Volume

Density, defined as mass per unit volume, provides insight into how compact the matter is. It’s calculated using:
- Density = Mass ÷ Volume.
Density has several implications:
- Material Identification: Different materials have unique densities, which can help identify substances.
- Floatation and Buoyancy: Objects with lower density than the fluid they’re placed in will float.
| Material | Density (g/cm³) |
|---|---|
| Water | 1 |
| Wood | 0.7 |
| Aluminum | 2.7 |
Weight: The Force of Gravity

While often confused with mass, weight is the force exerted on an object due to gravity. It depends on:
- Mass: The more mass, the greater the weight.
- Gravity: Weight varies with gravitational pull, which is not constant.
The relationship between mass and weight is:
Weight = Mass x Acceleration Due to Gravity.
👩🚀 Note: An astronaut’s mass doesn’t change in space, but their weight can be considered negligible due to the absence of significant gravitational pull.
Temperature: A Measure of Kinetic Energy

Temperature reflects the average kinetic energy of particles in a substance. Here’s why it’s important:
- Physical State Changes: Temperature determines whether matter is solid, liquid, or gas.
- Energy Transfer: Heat flows from high to low temperature regions, enabling heat engines to do work.
Applications of Properties of Matter

Understanding these properties isn’t just for the lab; it has real-world applications:
- Chemical Engineering: Designing processes where matter reacts, changes state, or is manipulated.
- Construction: Selecting materials based on their density, weight, and resistance to temperature.
- Aviation and Space Travel: Considering weight to mass ratio for fuel efficiency and structural integrity under gravitational forces.
The properties of matter, from mass to temperature, offer a lens through which we can understand and interact with the world around us. Whether you're studying these properties for academic purposes or applying them in practical settings, their understanding enables us to manipulate, predict, and explain natural phenomena.
Engaging with Matter: A Worksheet Approach

Here’s how you can engage with these properties using a worksheet:
- Mass: Use a balance to measure mass, then calculate density.
- Volume: Experiment with water displacement or mathematical calculations for different shapes.
- Weight: Measure the weight of objects in different environments or use spring scales.
- Density: Perform buoyancy experiments or identify unknown substances.
- Temperature: Investigate phase changes or heat transfer rates.
🔬 Note: When measuring volume, use a graduated cylinder for precision, especially with liquids.
Through worksheets, these fundamental properties become tangible, allowing students and enthusiasts alike to not only learn but also appreciate the science behind the everyday interactions with matter.
What is the difference between mass and weight?

+
Mass is a measure of the amount of matter in an object, which does not change with location. Weight, however, is the force exerted on that mass due to gravity, which can vary from place to place. For example, your mass stays the same on Earth or in space, but your weight changes due to gravitational differences.
How does temperature affect the properties of matter?

+
Temperature affects matter by altering its kinetic energy. As temperature increases, particles move faster, potentially changing the state of matter (e.g., from solid to liquid) or causing expansion or contraction in gases or solids. This influences density, volume, and other properties.
Why is understanding density important?

+
Density is key for material identification, predicting buoyancy, understanding how substances will interact in environments like air or water, and in industries like metallurgy and pharmaceuticals where precise composition matters.