Master Chemical Reaction Predictions Easily with This Worksheet

Understanding how chemicals react with one another is at the core of chemistry. From creating life-saving medications to developing cutting-edge technologies, knowing how to predict chemical reactions is essential for both academic success and practical applications. This blog post will guide you through the steps to easily master chemical reaction predictions with a handy worksheet designed to hone your skills.
Introduction to Chemical Reactions

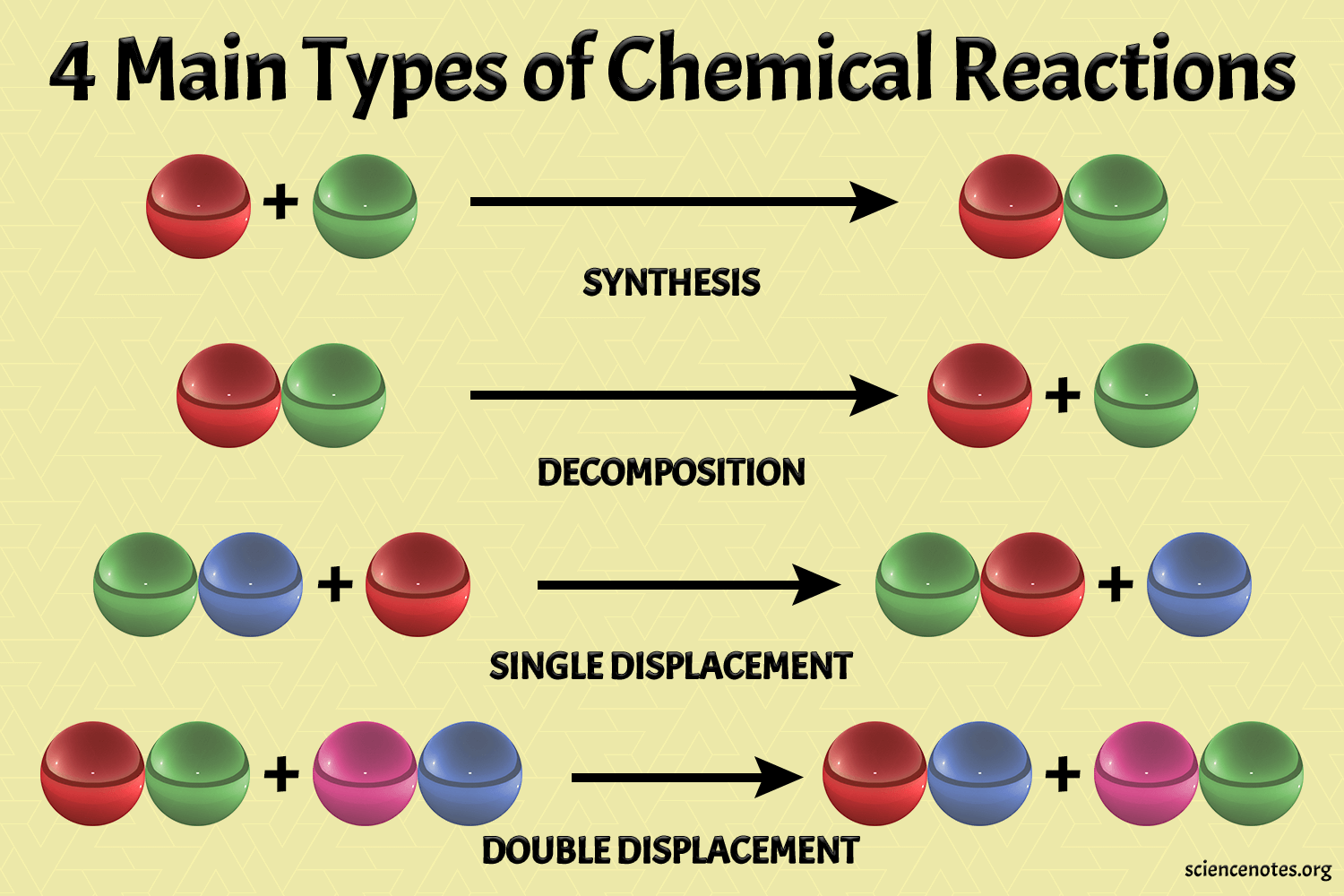
Chemical reactions involve the transformation of substances into different substances via the breaking and forming of chemical bonds. They can be classified into several types:
- Combination Reactions: Two or more substances combine to form a single substance.
- Decomposition Reactions: A single compound breaks down into two or more substances.
- Single Displacement Reactions: An atom or ion from one compound is displaced by another in the reactants.
- Double Displacement Reactions: Ions from two different compounds switch places, forming two new compounds.
- Combustion Reactions: A substance reacts with oxygen, often resulting in heat and light.
- Redox (Oxidation-Reduction) Reactions: Transfer of electrons between reactants.
It’s important to understand these types for predicting reactions effectively.
Using the Worksheet for Reaction Prediction

Our worksheet provides a structured approach to predict chemical reactions. Here’s how you can use it:
- Identify the Reactants: Begin by identifying all the reactants involved. Look at their names and chemical formulas.
- Determine the Reaction Type: Match the characteristics of your reactants with the types of reactions mentioned above. This helps in selecting the appropriate reaction equation.
- Predict the Products: Use your knowledge of chemistry to predict what new compounds might form. Here are some rules:
- For combination reactions, simply add the formulas together.
- In decomposition, break the compound apart.
- For single displacement, use the activity series to predict if displacement will occur.
- Double displacement reactions follow the rule of swapping ionic partners.
- Combustion reactions generally produce carbon dioxide and water (for organic compounds).
- For redox, balance the electron transfer.
- Balance the Equation: Ensure the number of atoms for each element is the same on both sides of the equation.
Example: Let’s predict the products for a simple combustion reaction:
| Reactants | Products |
|---|---|
| CH₄ (methane) + O₂ (oxygen) | CO₂ (carbon dioxide) + H₂O (water) |
Here, we identify the reactants, recognize it as a combustion reaction, predict CO₂ and H₂O as products, and then balance the equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
📝 Note: Always ensure that you write the correct chemical formulas when balancing equations. The subscripts (number of atoms in a compound) remain unchanged during balancing.
Advanced Tips for Prediction

Beyond basic reaction types, there are several advanced tips that can enhance your prediction skills:
- Consider Reaction Conditions: Temperature, pressure, and catalysts can influence the outcome.
- Understand Solubility Rules: Knowing which ions form soluble or insoluble compounds can help in predicting precipitate formation.
- Electronegativity: This can help in predicting which atom will gain or lose electrons in a redox reaction.
🔍 Note: While predicting, always check for patterns like the occurrence of common salts (NaCl, CaCO₃) or gas formation (H₂, CO₂, NH₃).
Wrapping Up

Mastering chemical reaction predictions involves a combination of understanding reaction types, recognizing patterns in reactants, and applying rules like solubility, electronegativity, and balancing. By utilizing the worksheet provided, you can systematically approach each reaction and improve your predictions over time. Remember, like any skill, practice is key, so keep working through different scenarios to enhance your proficiency.
What is the easiest way to identify a reaction type?

+
Start by examining the reactants and products, and check if they match any classic patterns like combination (A + B → C) or decomposition (A → B + C).
Why is balancing chemical equations important?

+
Balancing ensures the law of conservation of mass is maintained, as atoms cannot be created or destroyed in a chemical reaction.
Can reactions be predicted with 100% accuracy?

+
While rules can guide predictions, real-world reactions can be complex due to reaction conditions and other factors.