Unlock Energy Secrets: Potential Diagrams Answers Explained

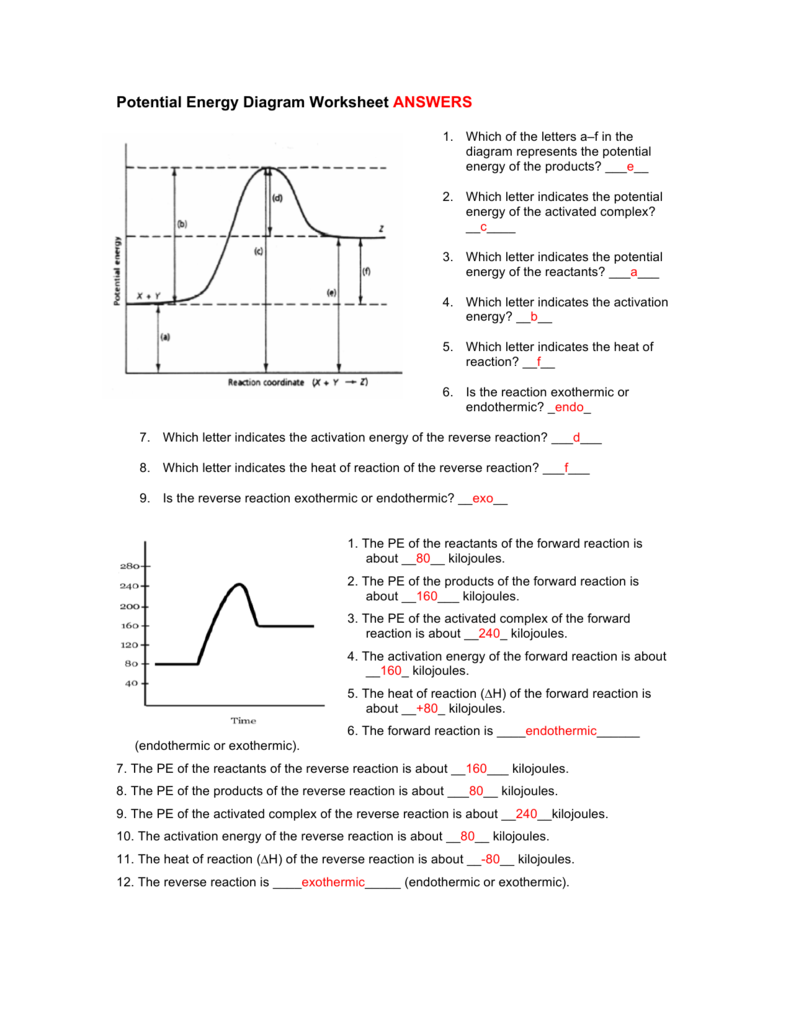
In the world of physics and chemistry, understanding how and why reactions occur is fundamental. Potential energy diagrams, often called reaction coordinate diagrams, are visual tools that depict the energy changes during a chemical reaction. They are crucial for comprehending the kinetics, thermodynamics, and mechanisms of reactions, providing insights into energy barriers, transition states, and the energy required or released during a reaction. This blog post will explore the intricacies of potential energy diagrams, explaining their components and how to interpret them effectively.
Understanding Potential Energy Diagrams

Potential energy diagrams are graphical representations where:
- The horizontal axis represents the reaction coordinate, a somewhat abstract measure of the progress of a reaction from reactants to products.
- The vertical axis indicates the potential energy of the system.
📝 Note: The terms "reactants," "products," and "transition state" are central to understanding how a chemical reaction progresses.
Key Components of a Potential Energy Diagram

- Reactants: These are the starting materials, and their energy level on the diagram is represented by a point at the left side of the curve.
- Products: The final state of the system, showing where the reaction ends, depicted at the right side.
- Transition State: The highest point on the curve, where the molecules are at their most unstable. This is where reactants are becoming products.
- Activation Energy (Ea): The energy difference between the reactants and the transition state. It's the energy barrier that must be overcome for the reaction to proceed.
- ΔH (Enthalpy Change): The energy change between reactants and products, indicating whether a reaction is exothermic (negative ΔH) or endothermic (positive ΔH).
Interpreting the Diagram

Here's how to interpret the information:
- Exothermic Reactions: The products are at a lower energy level than the reactants. The reaction releases energy, meaning ΔH is negative.
- Endothermic Reactions: The products are at a higher energy level than the reactants. Energy must be absorbed for the reaction to proceed, so ΔH is positive.
- Energy Barriers: The height of the energy barrier (Ea) determines the rate of reaction. A higher barrier means a slower reaction since it takes more energy for molecules to reach the transition state.
- Reaction Progress: The shape of the curve can indicate how the reaction progresses, including whether there are intermediates or alternative pathways.
Applications in Chemistry and Physics

Potential energy diagrams aren't just theoretical tools; they have practical applications:
- Catalysis: A catalyst lowers the activation energy, making the reaction occur more readily. This is depicted as a lower hump in the energy diagram.
- Enzyme Kinetics: Enzymes, being biological catalysts, also modify the energy landscape of biochemical reactions.
- Drug Design: Understanding how drugs interact with enzymes or receptors can be visualized through potential energy diagrams to predict binding affinities and reaction rates.
- Polymerization: In polymer chemistry, diagrams can show the energy changes when monomers form polymer chains.
Advanced Considerations
There are more advanced aspects to consider in interpreting these diagrams:
- Free Energy Diagrams: Incorporate entropy changes (ΔS) along with enthalpy changes to give a more complete picture of thermodynamic favorability.
- Energy Profiles with Multiple Transition States: Some reactions might involve multiple steps, each with its own transition state and activation energy.
- Effect of Temperature: Changes in temperature can alter the rate of reaction by affecting how often molecules have the necessary energy to overcome the activation barrier.
Learning to Draw Potential Energy Diagrams

Drawing potential energy diagrams is an essential skill for students and professionals in chemistry:
- Label Reactants and Products: Start with the energy levels of reactants and products.
- Determine Activation Energy: Plot the energy increase from reactants to transition state.
- Sketch the Energy Curve: The curve should start at the reactants, rise to the transition state, then fall to the product level.
- Include Key Points: Mark the transition state, reactants, products, and label Ea and ΔH.
- Consider Catalyst Effects: If needed, draw a second curve with a lower activation energy hump to show the effect of a catalyst.
🔍 Note: Always verify the details of the reaction mechanism, as not all reactions follow the simplistic single-step model depicted in basic diagrams.
The Importance of Potential Energy Diagrams

Understanding these diagrams provides several benefits:
- Reaction Insight: They give a visual depiction of how a reaction occurs, making it easier to understand the process.
- Problem-Solving: They are invaluable in solving kinetic problems, predicting reaction rates, and assessing the viability of synthetic pathways.
- Communication: Diagrams are a universal language in chemistry, allowing for clear communication of complex ideas.
- Education: They are a staple in educational settings, helping to explain and teach chemical reactions and energy concepts.
As we've journeyed through the explanation of potential energy diagrams, it's clear they are more than just theoretical constructs. They are vital for understanding chemical reactions at both the macroscopic and microscopic levels. By visually mapping the energy changes in a reaction, these diagrams help us predict the feasibility of reactions, the effects of catalysts, and even guide the design of new materials and drugs. They are indispensable tools in the chemist's arsenal, providing a means to make sense of the dynamic world of molecules and their interactions.
What is the difference between potential and kinetic energy in the context of a reaction?

+
Potential energy is the energy stored in a system due to the position or configuration of its components, whereas kinetic energy is the energy of motion. In a reaction, potential energy is what we track in potential energy diagrams, showing energy changes as bonds form or break. Kinetic energy, on the other hand, is related to the speed of molecules and affects collision frequency, but isn’t directly represented in these diagrams.
How does temperature affect a potential energy diagram?

+
Temperature doesn’t alter the energy levels on a potential energy diagram directly, but it affects the distribution of molecular kinetic energies. At higher temperatures, more molecules have the energy to overcome the activation barrier, which can be inferred from the diagram as an increased likelihood of reaching the transition state.
Can potential energy diagrams show if a reaction will occur spontaneously?

+
No, potential energy diagrams only show the energy changes associated with a reaction. Spontaneity is determined by Gibbs free energy (ΔG = ΔH - TΔS), which includes enthalpy (ΔH), entropy (ΔS), and temperature (T). However, diagrams can hint at spontaneity if ΔH is negative, suggesting an exothermic reaction, but they must be combined with entropy considerations for a complete understanding.
How can you use potential energy diagrams to determine the rate of a reaction?
+
The rate of a reaction is not shown directly on the diagram but can be inferred from the height of the activation energy barrier. A higher barrier typically indicates a slower reaction rate because fewer molecules will have the energy to surmount this barrier. Conversely, a lower barrier, or when a catalyst is involved, suggests a faster reaction rate.