Potato Osmosis Lab: Key Answers and Insights

In the dynamic realm of biology, understanding the behavior of cells under different conditions can provide profound insights into their functionality and adaptability. A common lab experiment that showcases this cellular behavior is the Potato Osmosis Lab. This experiment not only delves into the world of osmosis but also offers hands-on experience in biological processes and scientific inquiry. Here, we explore the key answers and insights from this experiment, providing clarity on how osmosis affects potato cells and what that reveals about cellular transport mechanisms.
Understanding Osmosis and Potato Cells

Osmosis is the process by which water molecules move from an area of higher concentration to an area of lower concentration through a semipermeable membrane. In the Potato Osmosis Lab, potato cells act as the semipermeable membrane, and water's movement illustrates this concept vividly:
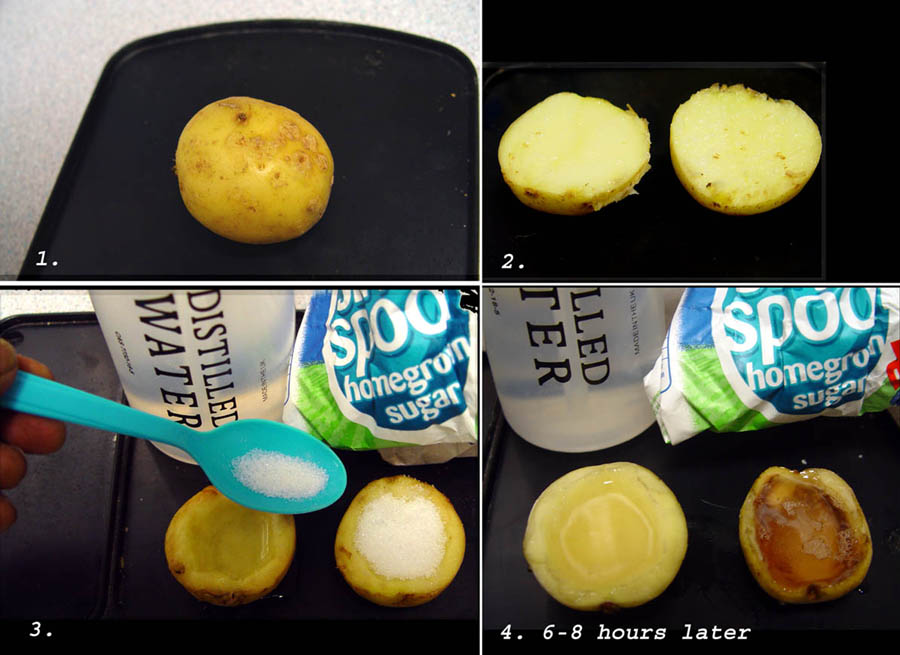
- Setup: Potatoes are cut into uniform pieces, and their initial mass is recorded. These pieces are then immersed in solutions of varying concentrations, typically from distilled water to saturated saline solutions.
- Observation: Over a set period, the changes in mass are monitored, giving us insights into water movement.
- Key Answer: The mass change in potatoes indicates the movement of water; potatoes lose mass in high-concentration solutions due to osmosis, drawing water out, and gain mass in hypotonic solutions where water enters the cells.
Setup and Execution

Performing this experiment involves several precise steps:
1. Preparing Potato Pieces

- Cut potatoes into equal-sized cubes to ensure uniformity.
- Weigh each piece and record the initial mass.
2. Solution Preparation

- Use a range of solutions, from pure water (0% solute concentration) to high salt or sugar solutions (e.g., 35% NaCl).
- Label each beaker with the solute concentration.
3. Experiment Execution

- Place potato pieces in different solutions.
- Leave the setup for a specified time, typically overnight.
- After the incubation period, weigh the potatoes again to measure the final mass.
🌿 Note: Ensure all potato pieces are the same size for fair comparison and control variables like temperature and exposure time.
Analyzing the Results

| Solution Concentration | Expected Mass Change | Reason |
|---|---|---|
| 0% (Pure Water) | Increase | Water enters the cells via osmosis since the external solution has less solute. |
| 10% Solute | Slight Increase or No Change | Cell's internal concentration might be close to this level, leading to a small or no osmotic gradient. |
| 20% Solute | Decrease | Water moves out of the potato cells as the external solution has a higher solute concentration. |
| 30% Solute | Significant Decrease | The osmotic gradient is large, causing substantial water loss from the cells. |

Insights from the Experiment

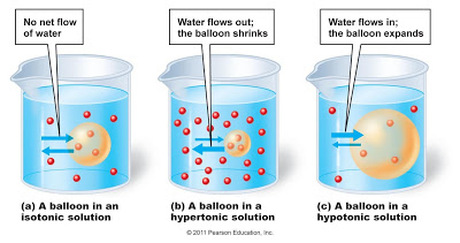
- Isotonic Solutions: These occur when the solute concentration inside and outside the cells is the same. In such conditions, potato pieces' mass remains relatively unchanged.
- Hypertonic and Hypotonic: If the solution outside is hypertonic (more solute than inside), water moves out, causing potatoes to lose mass. In hypotonic solutions, water moves in, leading to an increase in mass.
- Turgor Pressure: The experiment highlights how cells control their water balance. When water enters the cell, it creates turgor pressure, which is essential for plant cell structure and growth.
🧪 Note: This experiment also underscores the importance of turgor pressure in plants, which gives them rigidity and support.
Reflecting on the Potato Osmosis Lab, we can draw several conclusions about cellular transport and the interaction between cells and their environment:
- The demonstration of osmosis shows the selective permeability of cell membranes, allowing water but not large solute molecules to pass.
- It provides a practical way to observe how cells regulate their internal environment in response to external conditions.
- The lab serves as an excellent educational tool for students, illustrating fundamental biological concepts like osmosis, water potential, and the effects of different solute concentrations.
Through this experiment, we gain not just a better understanding of potato cells but a clearer picture of how life, at its microscopic level, manages its internal equilibrium. The Potato Osmosis Lab elucidates how even simple, everyday plants like potatoes can teach us about the complex, yet beautifully organized nature of biological systems.
What does osmosis tell us about plant cell function?

+
Osmosis shows how plant cells manage water movement to maintain their structure, turgidity, and support growth.
Why is it important to use different concentrations in the experiment?

+
Using various concentrations allows us to observe how the osmotic gradient affects water movement, demonstrating the sensitivity of cells to their environment.
Can we apply the principles of osmosis to human cells?
+
Absolutely. Osmosis is a universal biological principle. The balance of fluids and electrolytes in human cells is crucial for health, just as in plant cells.
What can go wrong in the Potato Osmosis Experiment?
+
Potential pitfalls include inaccurate solution preparation, inconsistent potato piece sizes, temperature variations, or insufficient time for osmosis to occur.
How does this experiment relate to real-world phenomena?
+
The principles observed in this lab apply to soil-water-plant relations, osmotic pressure in medical treatments, and food preservation techniques like pickling.



