Polyatomic Ions Worksheet: Answers Explained Clearly

Understanding Polyatomic Ions: A Comprehensive Guide

Polyatomic ions are groups of atoms covalently bonded together that carry a net electrical charge. These ions are pivotal in understanding chemical reactions, especially when it comes to the formation of compounds, their solubility, and their reactivity. This guide is intended to help you better comprehend polyatomic ions through detailed explanations and practical examples.
What are Polyatomic Ions?

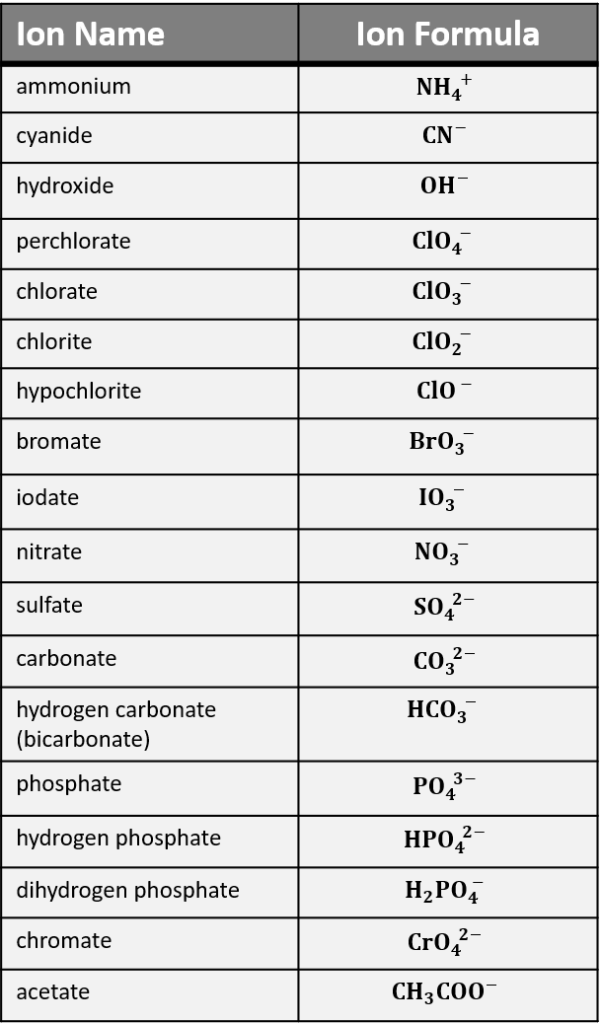
Polyatomic ions consist of two or more atoms that have an overall electric charge. Unlike monatomic ions, which are composed of a single atom, polyatomic ions can be either positively or negatively charged, creating a wide range of ions with various charges. Here are some common examples:
- Hydroxide: OH⁻
- Nitrate: NO₃⁻
- Sulfate: SO₄²⁻
🔍 Note: Knowing the charges of common polyatomic ions is essential for balancing chemical equations and predicting the properties of compounds.
Naming Polyatomic Ions

The naming of polyatomic ions follows specific rules:
- If the ion ends in -ite, -ate, or -ide, it often indicates the presence of oxygen. For example, nitrate (NO₃⁻) contains three oxygen atoms.
- The suffix -ate typically signifies the presence of more oxygen than the -ite version.
- If the ion ends with -ium, it indicates a positive charge. For example, ammonium (NH₄⁺).
| Ion | Formula | Charge |
|---|---|---|
| Phosphate | PO₄³⁻ | 3- |
| Sulfite | SO₃²⁻ | 2- |
| Ammonium | NH₄⁺ | 1+ |
Applications in Chemical Equations

Polyatomic ions play a crucial role in balancing chemical equations:
- Identify the ions: Understand which ions are involved in the reaction.
- Balance charges: Ensure that the total charge on both sides of the equation is equal.
- Balance atoms: Make sure that the number of atoms of each element is balanced on both sides.
🧪 Note: In double-displacement reactions, knowing the solubility of polyatomic ions can help predict the precipitation of salts.
Worksheet and Solutions
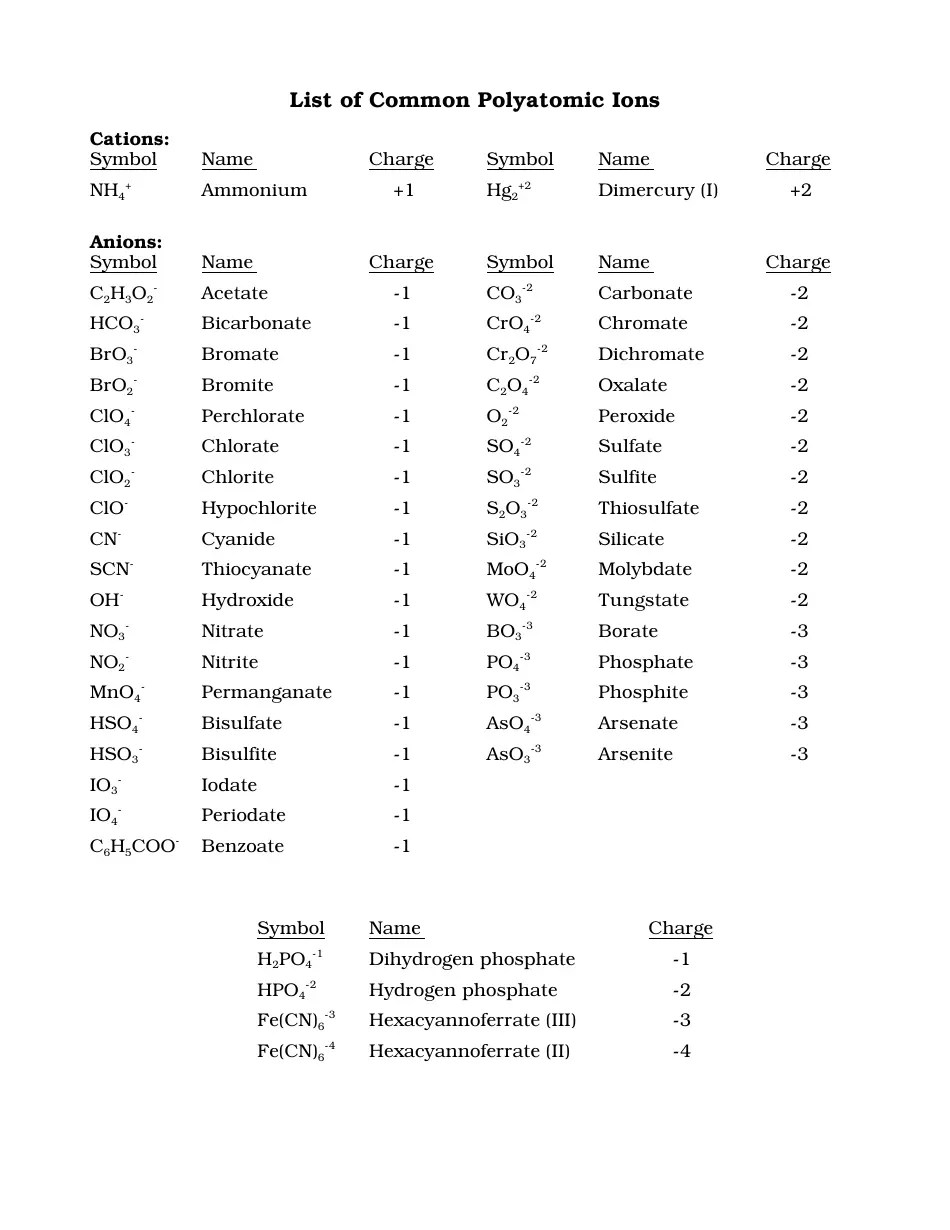
Here's a sample worksheet to reinforce your understanding of polyatomic ions:
Problem 1: Write the formula for each of the following ions:
- Carbonate
- Cyanide
- Acetate
- Carbonate: CO₃²⁻
- Cyanide: CN⁻
- Acetate: CH₃COO⁻
Problem 2: Identify the charges on the following polyatomic ions:
- Permanaganate
- Perchlorate
- Permanaganate: 1-
- Perchlorate: 1-
Problem 3: Write the chemical formula for the compound formed between sodium and phosphate ions. Answer: Na₃PO₄
📝 Note: Practice writing and balancing equations with polyatomic ions can significantly improve your chemical problem-solving skills.
Common Mistakes to Avoid

When working with polyatomic ions, common pitfalls include:
- Overlooking the charge of the ion, which affects the formula.
- Confusing similar-sounding ions (like nitrite and nitrate).
- Incorrectly balancing charges when forming compounds or balancing equations.
Final Thoughts

Polyatomic ions are fundamental to understanding chemical behavior and composition. By familiarizing yourself with their charges, names, and formulas, you equip yourself with the tools necessary to tackle more complex chemistry topics. Practice is key, and engaging with worksheets and problem sets as outlined in this guide will help solidify your understanding. Whether you're looking to advance in academic studies or just to have a better grasp of chemical concepts, the knowledge of polyatomic ions will prove invaluable.
What is the difference between a polyatomic ion and a monatomic ion?

+
A polyatomic ion consists of two or more covalently bonded atoms with a net charge, while a monatomic ion is composed of a single atom with a charge.
How do you balance chemical equations involving polyatomic ions?

+
You balance chemical equations by ensuring that the total charge and the number of atoms for each element are the same on both sides of the equation. Polyatomic ions should be treated as single units when balancing.
Can polyatomic ions exist independently in solution?
+
Yes, polyatomic ions can exist independently in solution. They maintain their charge and structure, often dissociating in water to form ions like OH⁻ or NO₃⁻.