Polyatomic Ions Formulas Worksheet: Master Chemistry Easily
In the vast world of chemistry, understanding polyatomic ions is pivotal for mastering topics like chemical reactions, nomenclature, and the principles governing the behavior of substances at an atomic level. Polyatomic ions, being groups of atoms that carry a charge, are encountered frequently in various chemical compounds. This comprehensive guide not only introduces the concept but also provides a detailed Polyatomic Ions Formulas Worksheet, ensuring that both novice and seasoned chemistry enthusiasts can achieve proficiency in this area.
Why Learn Polyatomic Ions?

The importance of polyatomic ions stretches across various scientific disciplines:
- Accurate Nomenclature: Proper naming of chemical compounds relies heavily on the knowledge of polyatomic ions.
- Chemical Reaction Balancing: Their understanding aids in writing balanced chemical equations accurately.
- Industrial Applications: Many industrial processes involve compounds with polyatomic ions, from pharmaceuticals to agriculture.
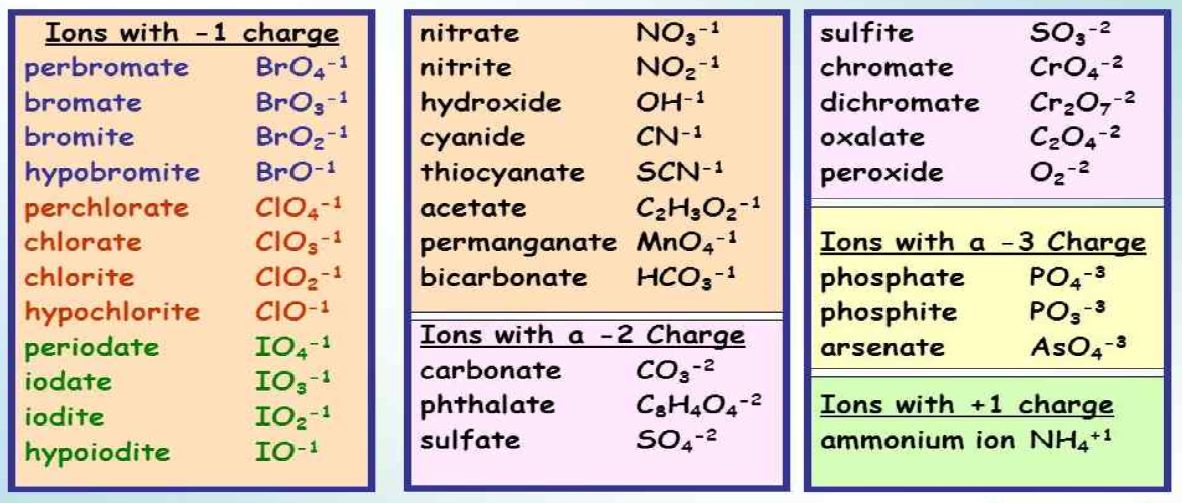
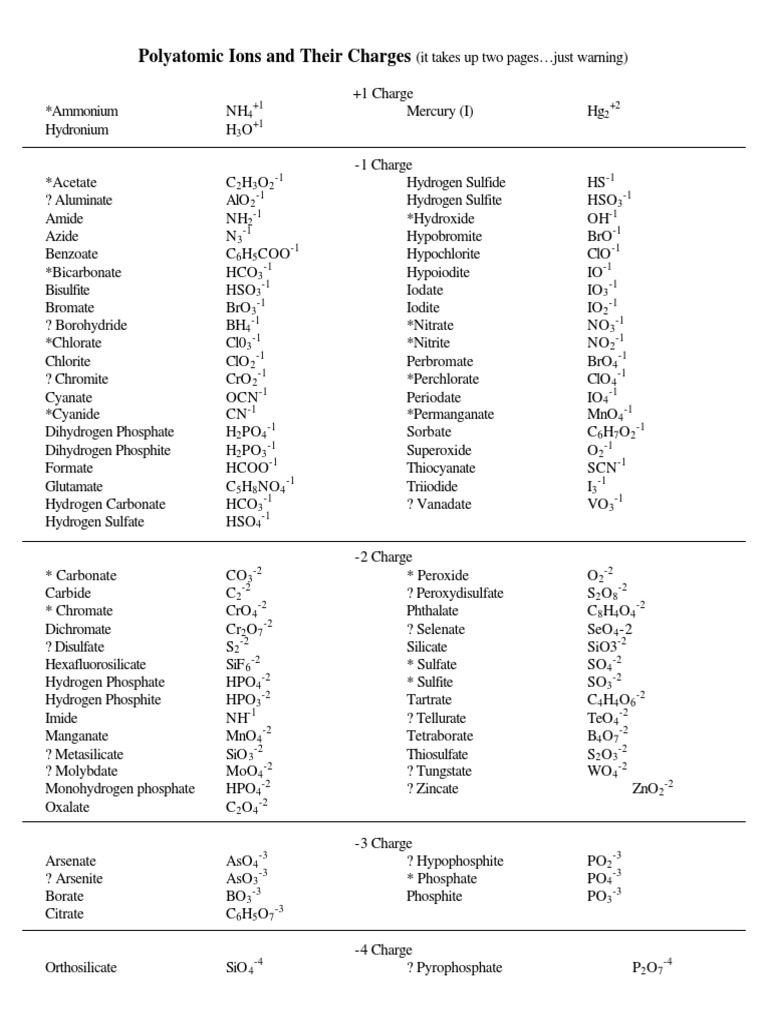
Essential Polyatomic Ions

Below is a table listing some common polyatomic ions, their formulas, and charges:
| Ion Name | Formula | Charge |
|---|---|---|
| Ammonium | NH4+ | +1 |
| Acetate | C2H3O2- | -1 |
| Hydroxide | OH- | -1 |
| Nitrate | NO3- | -1 |
| Phosphate | PO43- | -3 |
| Sulfate | SO42- | -2 |
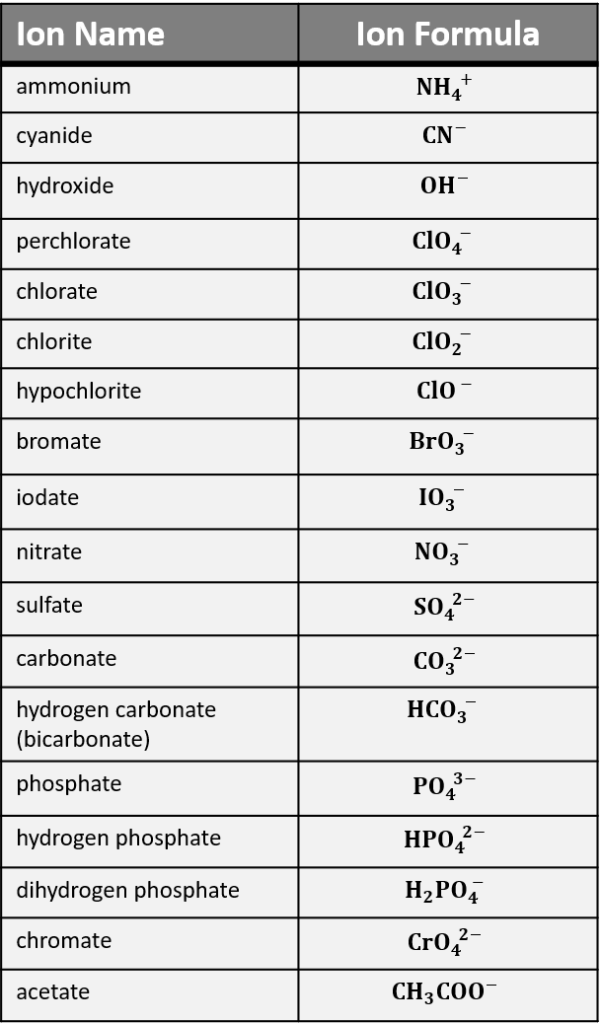
📝 Note: It's essential to memorize these ions as they often appear in chemical equations and formulas.
How to Master Polyatomic Ions?
To become adept at handling polyatomic ions in chemistry, consider the following steps:
Memorization Techniques
- Flashcards: Create flashcards with the ion name on one side and its formula on the other. Use colors or mnemonics for better retention.
- Quiz Yourself: Regularly test your knowledge with quizzes. Online platforms offer interactive tools for this purpose.
- Patterns Recognition: Look for common patterns in the ions’ structures, charges, and names.
Practical Applications
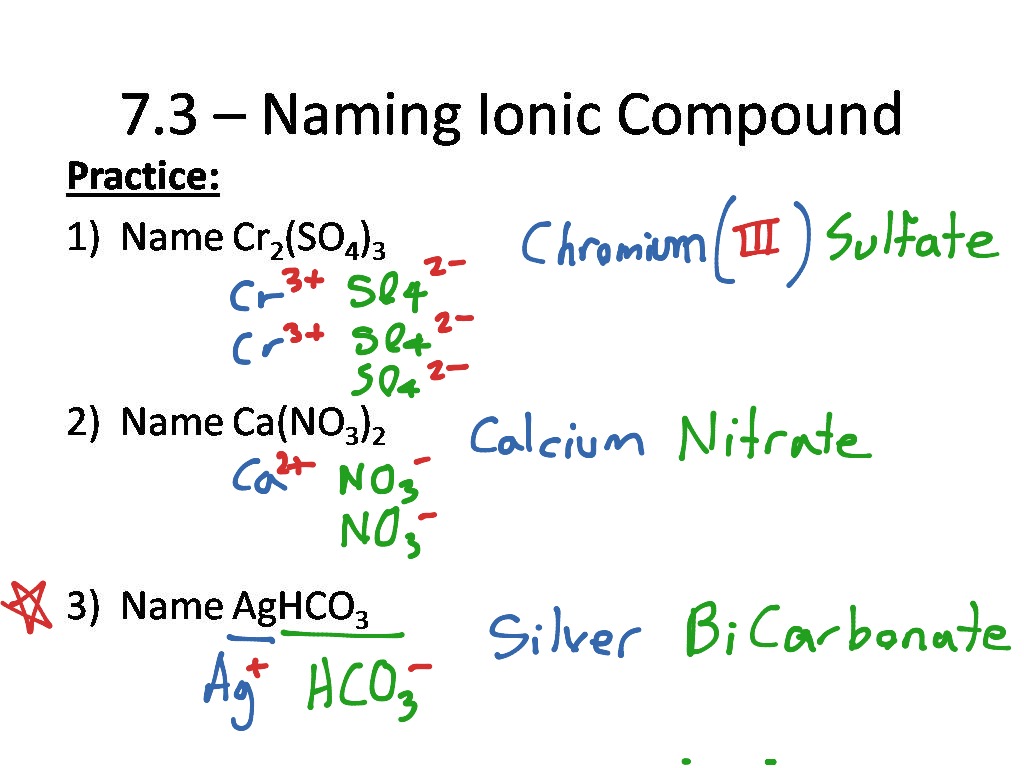
- Compound Formation: Write out different salts using the ions listed above. For instance, what compounds could you form with ammonium?
- Equation Balancing: Practice balancing chemical equations that involve polyatomic ions.
- Chemistry Software: Use educational software or apps to simulate reactions with polyatomic ions.
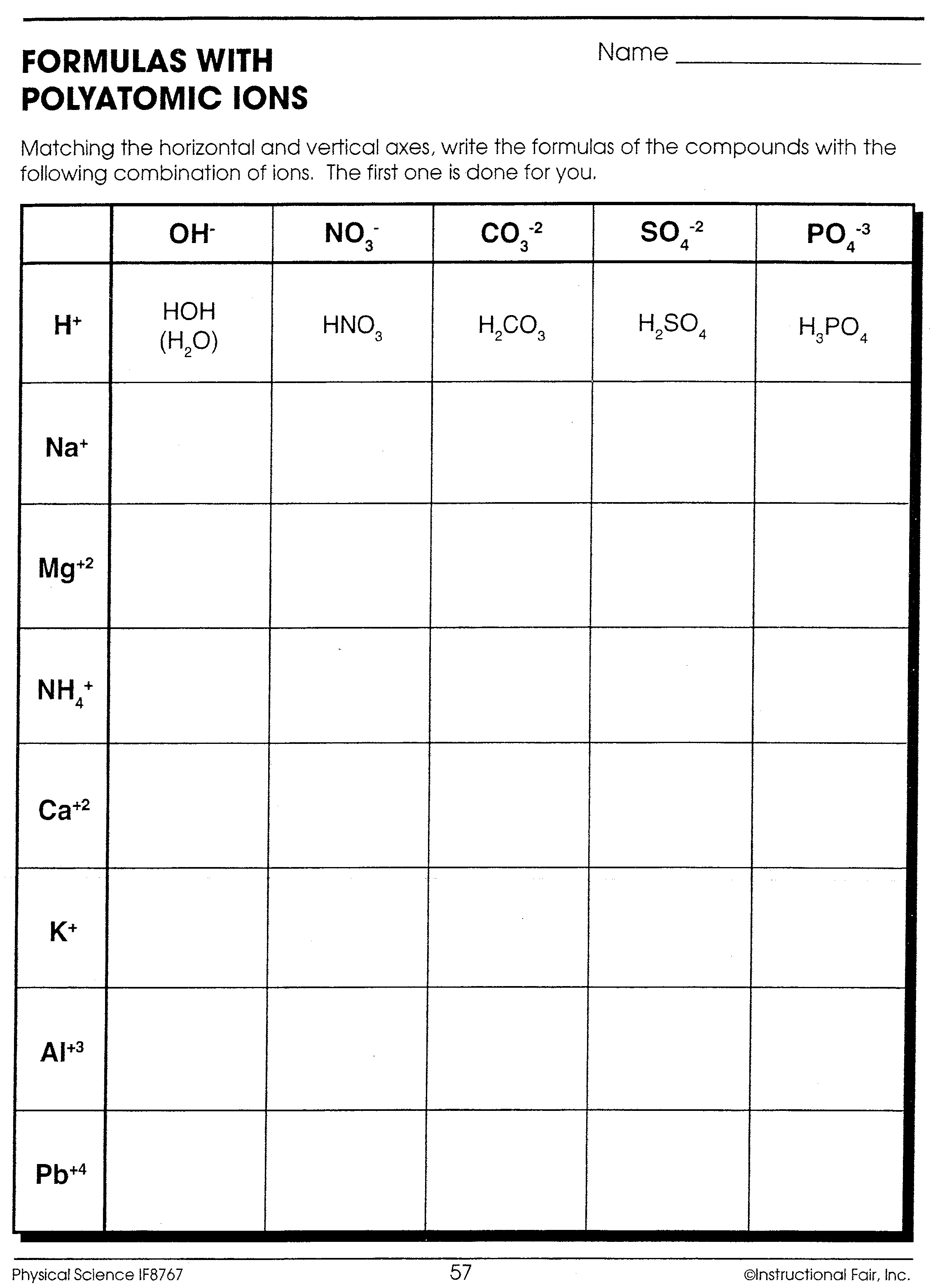
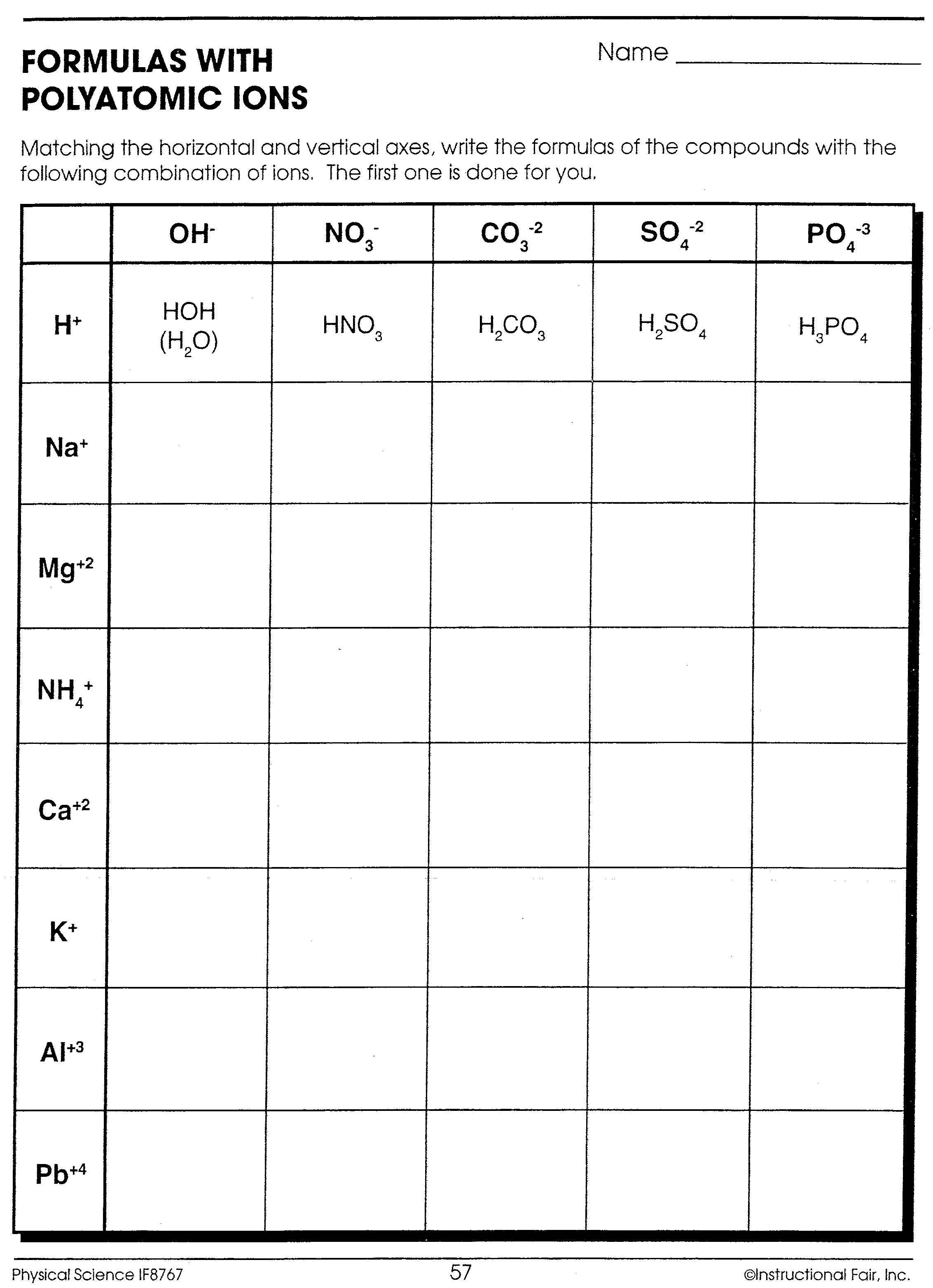
Polyatomic Ions Formulas Worksheet
Here is an example of exercises you might encounter when learning about polyatomic ions:
Exercise 1: Write the chemical formula for each of the following compounds:
- Sodium nitrate
- Calcium hydroxide
- Aluminum sulfate
Answers:
- NaNO3
- Ca(OH)2
- Al2(SO4)3
Exercise 2: Balance the following chemical equation:
Al(OH)3 + H3PO4 → AlPO4 + H2O
Answer:
Al(OH)3 + H3PO4 → AlPO4 + 3H2O
⚠️ Note: When balancing equations, ensure that the charge and atom counts are equal on both sides.
Wrapping Up

Through this guide, we’ve explored the essential polyatomic ions that form the backbone of inorganic chemistry. From memorization techniques to practical applications, we’ve laid out a roadmap for mastering these ions. Remember, regular practice and the use of educational tools can significantly enhance your learning experience. Keep practicing with our Polyatomic Ions Formulas Worksheet, and you’ll soon find yourself confidently navigating through chemical compounds and equations with ease.
Why are polyatomic ions important in chemistry?

+
Polyatomic ions are crucial in chemistry because they appear in many common compounds, affecting how we name, write, and balance chemical equations.
How can I memorize polyatomic ions effectively?

+
Use techniques like flashcards, mnemonics, recognizing patterns in ion names and charges, and practical application through equation balancing.
Are there any online resources to learn polyatomic ions?

+
Yes, there are many educational websites, apps, and tools that offer interactive quizzes and simulations for learning polyatomic ions.