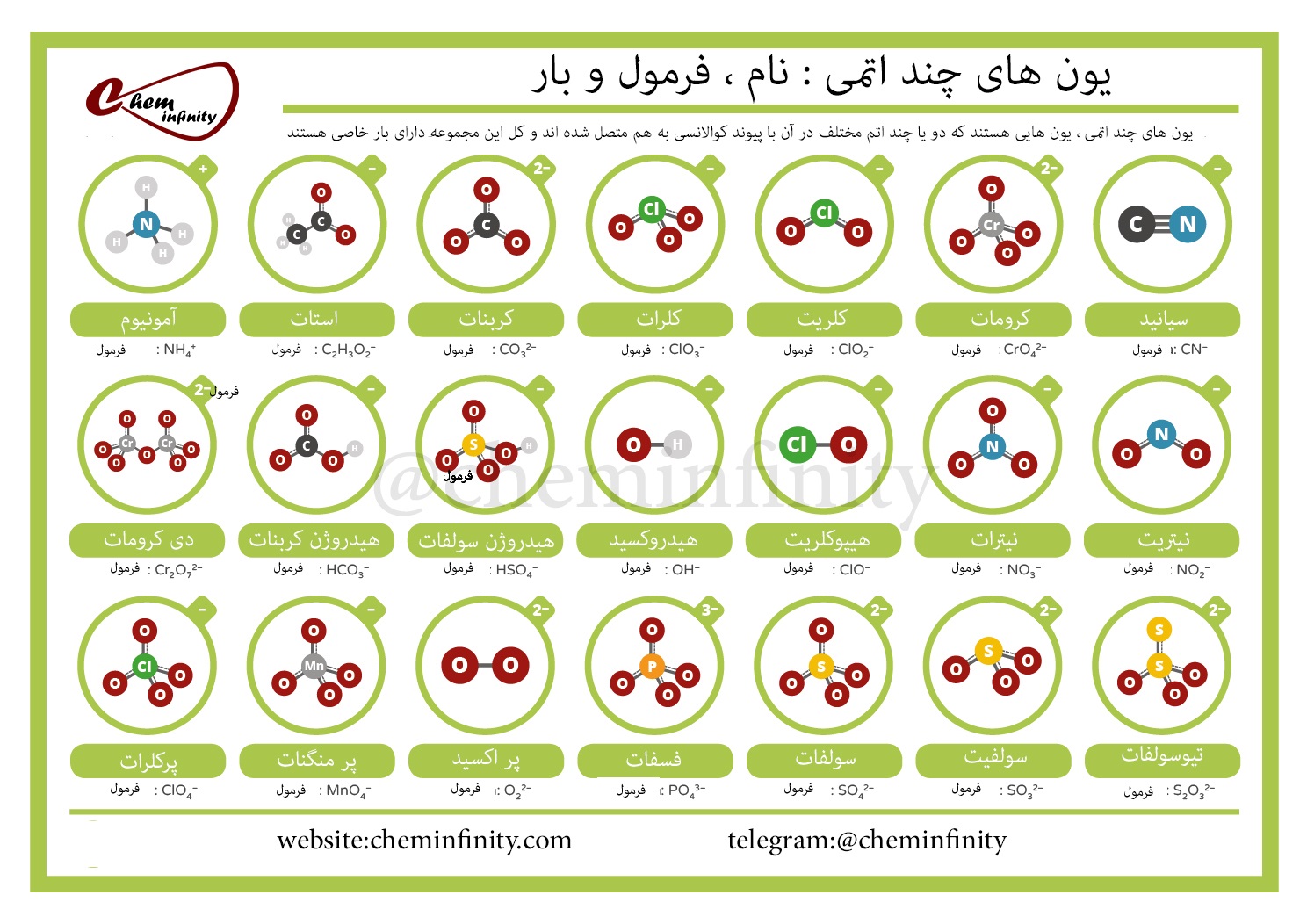
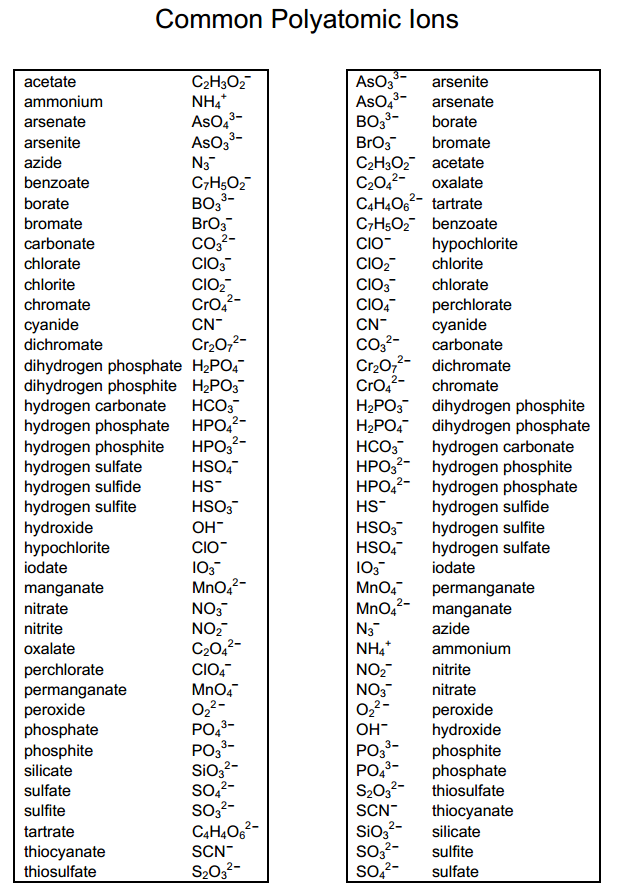
5 Essential Polyatomic Ions for Chemistry Students

In chemistry, polyatomic ions are critical for understanding the complexities of compound formation and the behavior of elements in solutions. These ions are groups of atoms covalently bonded together, carrying a net electric charge. They are not only significant in academic studies but also play essential roles in environmental chemistry, pharmaceuticals, and industrial processes. This post aims to guide students through five essential polyatomic ions they will frequently encounter in their studies.
1. Ammonium (NH4+)
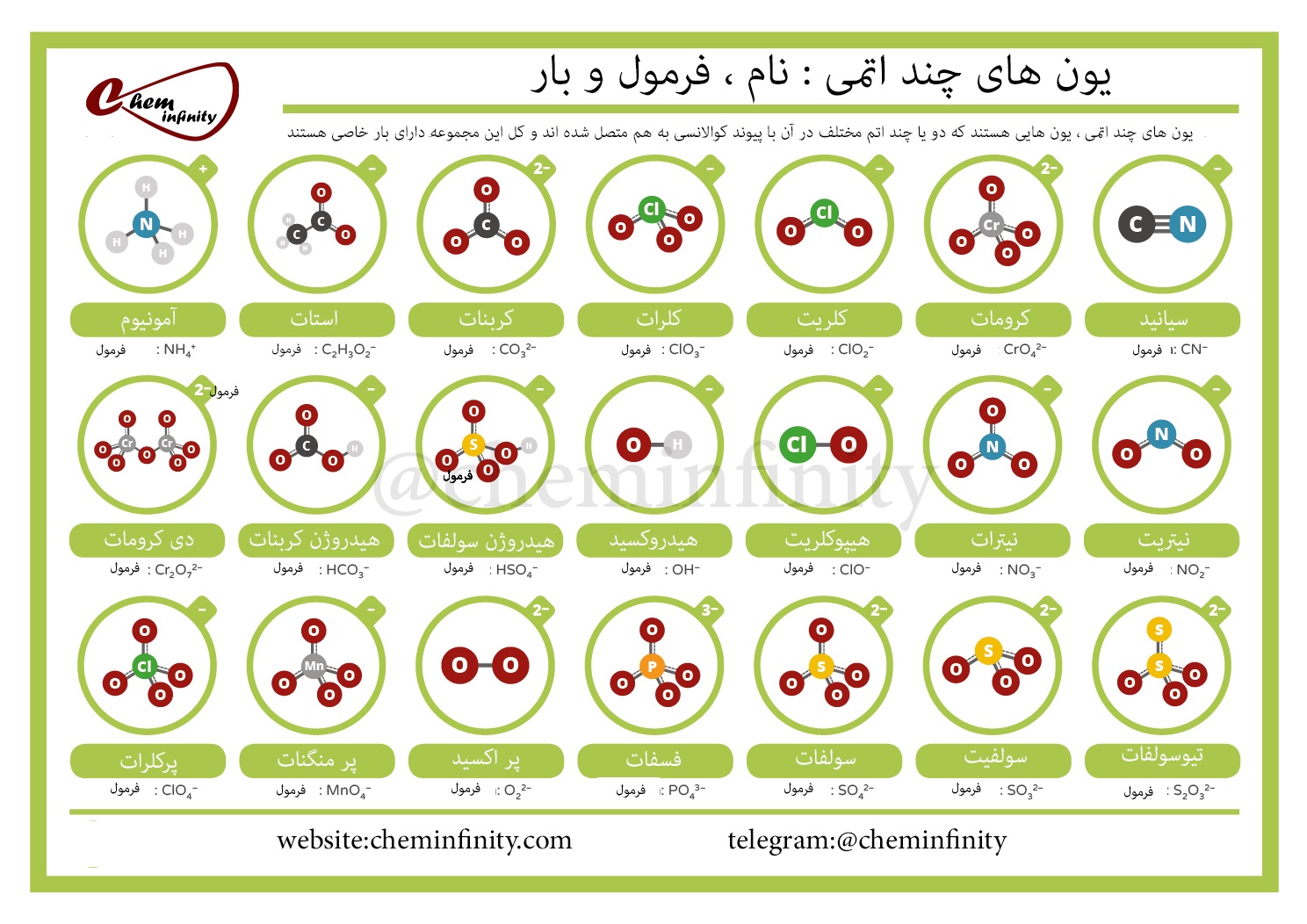
The ammonium ion is one of the most recognized polyatomic ions, comprising one nitrogen atom surrounded by four hydrogen atoms. Its chemical formula is NH4+.
- Formation: Ammonium ions are formed when ammonia gas (NH3) dissolves in water, capturing an additional proton to become ammonium.
- Common Uses:
- Fertilizers (ammonium sulfate, ammonium nitrate).
- In explosives (ammonium nitrate).
- Water treatment to control pH and in the process of nitrification in soil.
Chemical Properties

Ammonium ions are unique because they behave similarly to Group 1A elements in terms of chemical reactions due to their +1 charge:
- It forms compounds like ammonium chloride (NH4Cl) which is used in various chemical applications.
- Ammonium ions can decompose to give off ammonia gas when heated, which is a characteristic reaction.
⚗️ Note: When dealing with ammonium compounds, always ensure good ventilation as ammonia gas is highly irritating to the respiratory system.
2. Nitrate (NO3-)

The nitrate ion, with the formula NO3-, is a key component in numerous natural and industrial processes.
- Formation: It forms when nitrogen-containing compounds are oxidized, often through bacterial action in soil or lightning in the atmosphere.
- Common Uses:
- As a source of nitrogen for plant growth in fertilizers.
- In explosives like ammonium nitrate and nitroglycerin.
- In food preservation, especially curing meats.
Chemical Properties

- Nitrate ions are stable in solution but can be reduced to nitrite or further to nitrogen gas, especially in anaerobic conditions.
- They are key players in the nitrogen cycle, being the end product of nitrification.
💡 Note: Be aware of nitrate pollution in groundwater, as high levels can lead to health issues like methemoglobinemia in infants.
3. Sulfate (SO42-)
The sulfate ion, SO42-, is vital for understanding both environmental chemistry and industrial applications.
- Formation: Sulfate ions form when sulfur-containing compounds are oxidized, like sulfur dioxide from industrial processes or sulfide from water treatment plants.
- Common Uses:
- In detergents as sodium lauryl sulfate.
- As a coagulant in the treatment of water to remove impurities.
- In fertilizers to provide sulfur nutrition to plants.
Chemical Properties
Sulfate ions:
- Can form insoluble salts with certain metals like calcium (gypsum) and barium (barite), which have various uses in industry.
- They are involved in the sulfur cycle, being a final form of oxidized sulfur in the environment.
4. Hydroxide (OH-)

The hydroxide ion, OH-, is fundamental in acid-base chemistry.
- Formation: Hydroxide ions are formed when a base (like sodium hydroxide) dissolves in water, releasing OH- ions.
- Common Uses:
- In cleaning agents and drain cleaners for their caustic properties.
- As a reagent in titration processes to determine pH or to neutralize acids.
- In the manufacture of paper, textiles, and soaps.
Chemical Properties

Hydroxide ions:
- React with acids to form water and salt in a neutralization reaction.
- Are responsible for the alkaline properties of bases.
- In highly concentrated solutions, can act as a strong base.
5. Phosphate (PO43-)

Phosphates are essential in biological systems, industrial applications, and environmental cycles.
- Formation: Phosphorus, often mined as phosphate rock, is processed to produce phosphorus-based compounds like phosphoric acid.
- Common Uses:
- In fertilizers for plant nutrition.
- In animal feed to prevent osteoporosis.
- In food and beverages as buffering agents and preservatives.
Chemical Properties

- Phosphates can form several acids with varying degrees of acidity (e.g., phosphoric acid).
- They are involved in energy transfer within cells (ATP).
- In aquatic environments, excess phosphates can lead to eutrophication.
In summary, understanding these five polyatomic ions not only enhances your grasp of chemical reactions but also connects chemistry to everyday life, from fertilizers to medications. Each ion has distinct properties, applications, and environmental implications, making them indispensable in various fields of study and applications.
What is the most common use for the ammonium ion in everyday life?

+
The most common use for the ammonium ion in everyday life is in fertilizers, providing plants with necessary nitrogen for growth.
Why are nitrate ions important in the nitrogen cycle?

+
Nitrate ions are crucial as they represent the end product of nitrification in the soil, where bacteria convert ammonium and organic nitrogen into nitrate, making nitrogen available for plants.
How can sulfate ions contribute to water treatment?

+
Sulfate ions, particularly when forming aluminum sulfate or ferric sulfate, act as coagulants in water treatment to remove impurities by forming precipitates.



