5 Answers to Understand Planck's Constant Worksheet

In the realm of quantum mechanics, Planck's constant stands as a fundamental element, often bewildering students and even seasoned scientists with its profound implications. This unique constant, denoted by h , is not only the linchpin that bridges the classical and quantum worlds but also a key to understanding many phenomena in modern physics. Let's delve into some of the most frequently asked questions about Planck's constant to demystify this pivotal concept.
What is Planck’s constant?
Planck’s constant, named after the German physicist Max Planck, is a physical constant that relates the energy carried by a photon to its frequency. Its value is approximately ( 6.626 \times 10^{-34} \text{Js} ). Here are some fundamental points about Planck’s constant:
- It signifies the transition from classical to quantum mechanics.
- It plays a crucial role in determining the smallest possible energy difference in quantum systems.
- Planck’s constant is used in numerous equations, including the energy of a photon ( E = h\nu ).
Why is Planck’s constant significant in quantum mechanics?

Quantum mechanics would be incomprehensible without Planck’s constant:
- It quantizes the energy of particles, leading to phenomena like discrete energy levels in atoms.
- The smallness of ( h ) explains why quantum effects are not observable on macroscopic scales.
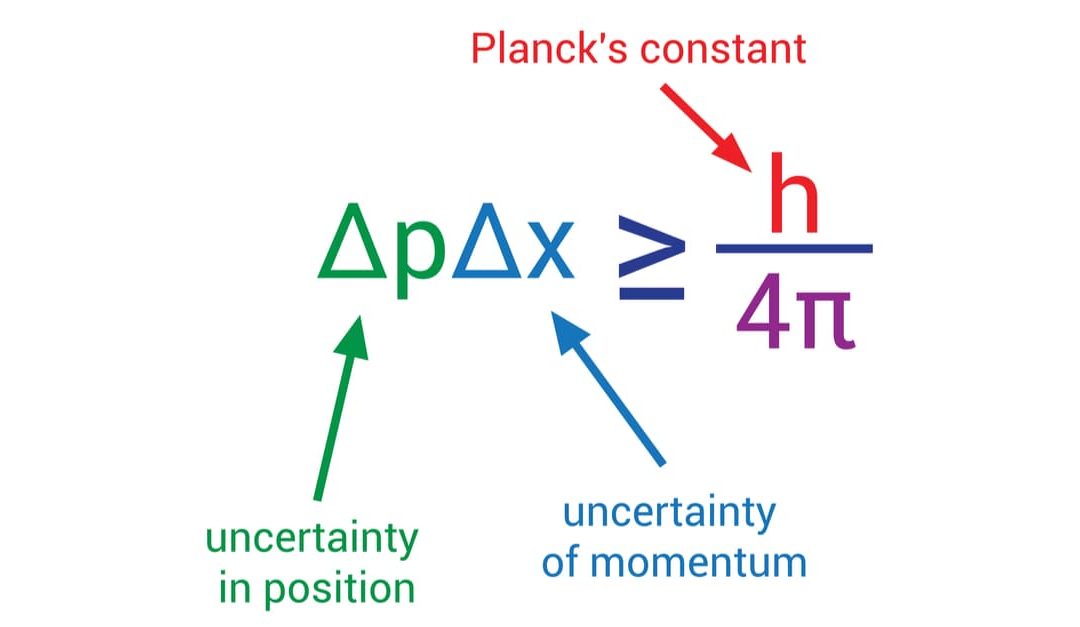
- It’s involved in the Heisenberg uncertainty principle, which sets a limit on the precision with which position and momentum can be known simultaneously.
How does Planck’s constant relate to the energy of photons?

The energy ( E ) of a photon is directly proportional to its frequency ( \nu ) through Planck’s constant:
- The relationship is given by ( E = h\nu ).
- The units for ( E ) are in joules (J), frequency ( \nu ) is in Hertz (Hz), and Planck’s constant ( h ) has units of J s (joule-seconds).
- This equation signifies that energy can only be exchanged in discrete packets or quanta.
How do we measure Planck’s constant experimentally?
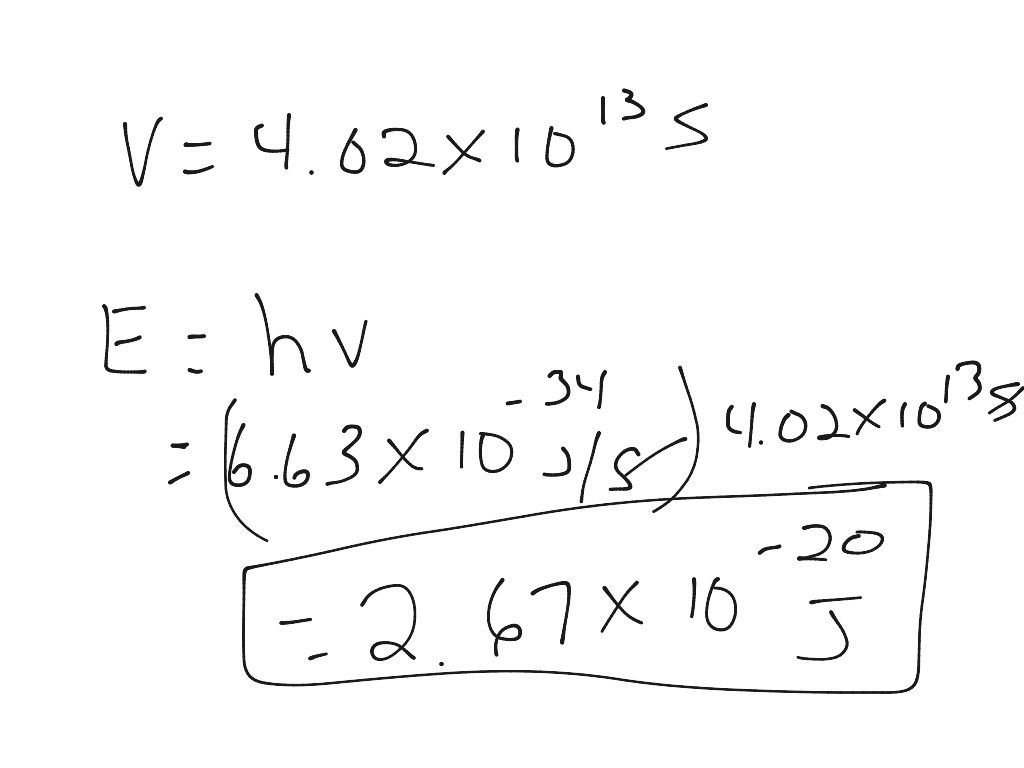
Here are some notable methods:
- Photoelectric Effect: By measuring the stopping potential and frequency at which electrons are emitted from a metal surface, Planck’s constant can be determined.
- Atomic Spectra: The spacing of atomic spectral lines can be used to deduce Planck’s constant.
- Josefson Voltage-Frequency Relation: In the context of superconducting junctions, the relationship between voltage and frequency provides a way to measure ( h ).
🔬 Note: While these methods provide experimental values for Planck's constant, the value obtained can vary slightly due to precision limitations in measurement equipment.
Can Planck’s constant change or is it truly constant?

In standard physics models:
- Planck’s constant is considered immutable, essential to the fabric of our understanding of the universe.
- However, some speculative theories in quantum gravity or cosmology suggest that fundamental constants might not be truly constant, varying ever so slightly over cosmological timescales.
- Current experiments have placed strict limits on how much Planck’s constant might vary, showing no significant changes.
In conclusion, Planck's constant is an integral part of quantum mechanics, symbolizing the quantization of energy and the bridge between the microscopic and macroscopic worlds. By understanding the role and implications of Planck's constant, we gain insight into why the universe behaves the way it does at its most fundamental levels. Its constancy and significance in various experimental methods showcase its indispensable place in modern physics, leaving us with a deep appreciation for the mysteries and beauty of our quantum universe.
Why did Planck introduce his constant?

+
Max Planck introduced his constant in 1900 while attempting to understand the spectral distribution of black-body radiation. It was necessary to resolve an issue known as the ultraviolet catastrophe, where classical theory predicted infinite energy, which was clearly false. Planck proposed that energy is radiated or absorbed in discrete packets or quanta, and the value of these quanta is proportional to the frequency through his constant ( h ).
What are the units of Planck’s constant?

+
Planck’s constant has units of joule-seconds (J s), sometimes written as kg m2/s in the SI system. In some calculations, the reduced Planck’s constant ( \hbar = \frac{h}{2\pi} ) is used, having units of joule-seconds or electronvolts per terahertz (eV/THz).
What are some practical applications of Planck’s constant?

+
Planck’s constant underpins many applications in modern technology and science:
- Laser technology and LED displays rely on the precise energy of photon emissions.
- Quantum computing relies on the quantization of energy levels.
- In medical imaging, understanding the interaction of photons with matter hinges on Planck’s constant.
- Photoelectric cells and solar energy conversion involve processes described by Planck’s constant.



