5 Tips for Mastering Phet Reversible Reactions Worksheet

Reversible reactions are fundamental to understanding many chemical processes, from industrial applications to biological systems. If you're looking to master the concept, using resources like the PhET Interactive Simulations' Reversible Reactions worksheet can significantly enhance your learning. Here are five tips to guide you through effectively navigating and mastering this educational tool:
1. Understand the Basics Before Diving In

Before you start experimenting with the PhET simulation, familiarize yourself with the fundamentals of reversible reactions. Here’s what you should know:
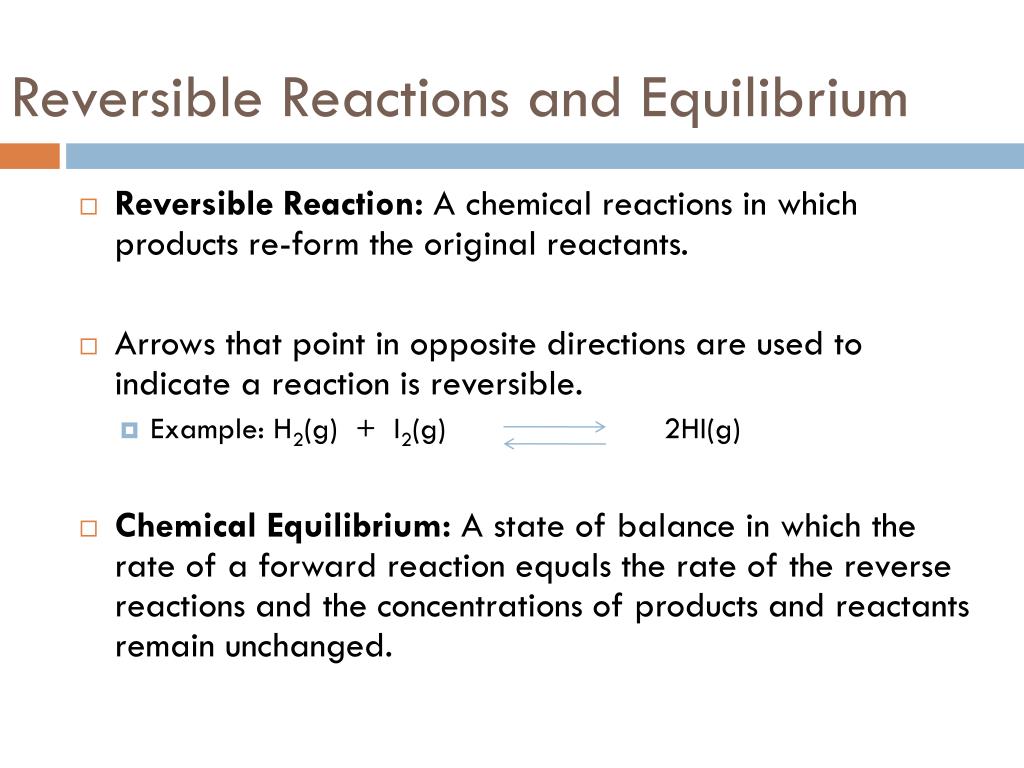
- Definition: Reversible reactions are chemical reactions where reactants form products that can revert back to reactants under the same conditions.
- Equilibrium: The state where the rate of the forward reaction equals the rate of the reverse reaction, leading to no net change in the concentration of reactants or products.
- Le Chatelier’s Principle: This principle explains how a system at equilibrium responds to changes in temperature, pressure, or concentration to restore balance.

2. Experiment with Variables

PhET’s Reversible Reactions simulation allows you to adjust different variables to see how they impact the reaction:
- Change the temperature and observe how it affects the equilibrium state.
- Adjust the concentration of either reactants or products and note the shift in the equilibrium position.
- Pressure changes in gaseous reactions can also be explored to understand the response as per Le Chatelier’s Principle.
🔬 Note: Experiment slowly, observing the change in colors or the shift in equilibrium. This will help you understand each variable’s influence clearly.
3. Use the Worksheet to Guide Your Exploration

The PhET worksheet provides structured tasks to guide your learning. Here’s how to make the most of it:
- Follow the worksheet questions step-by-step. Each question is designed to direct you to explore certain aspects of the simulation.
- Make sure to write down your observations for each step. This not only helps in memorizing but also in analyzing the reaction conditions.
- If a question requires calculation or prediction, try to perform these independently before checking with the simulation.
| Worksheet Section | Action | Learning Objective |
|---|---|---|
| Introduction to Reversible Reactions | Read and understand | Foundational knowledge of reversible reactions |
| Experimenting with Variables | Run simulation with different settings | Understand equilibrium shifts |
| Questions | Answer questions using simulation results | Application of concepts learned |

4. Record and Analyze Data
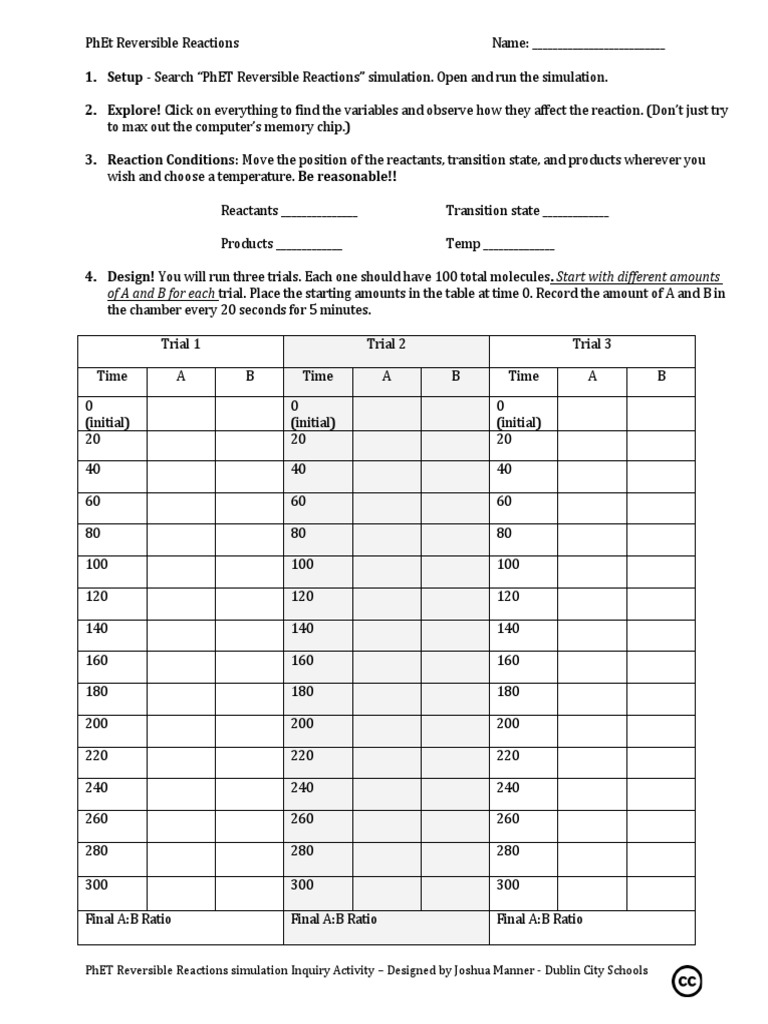
Data recording is crucial when working with scientific simulations:
- Keep a detailed lab notebook or digital document to log the initial conditions, changes made, and the resulting equilibrium states.
- Graph changes in concentrations over time to visualize how the system reaches equilibrium.
- Analyze your data to understand trends and verify concepts like Le Chatelier’s principle in action.
📊 Note: Analysis of data helps in recognizing patterns, which is key for mastering chemical reactions.
5. Review and Reflect

After working through the worksheet and simulation:
- Revisit your initial notes and any predictions you made to compare with the simulation results. Reflect on where your predictions were correct or incorrect.
- Summarize your findings in a few key points or conclusions. Focus on what you learned about the effects of different conditions on reversible reactions.
- Discuss with peers or mentors. This can provide different perspectives and reinforce your understanding through explanations to others.
Mastering the PhET Reversible Reactions worksheet is not just about completing tasks but understanding the dynamics of chemical equilibrium. By following these tips, you'll gain a deeper appreciation of the delicate balance in chemical systems, how they respond to stress, and the real-world applications of these principles. Remember, the more you engage with the simulation, the better you'll grasp these essential chemical concepts.
How do I know when the reaction has reached equilibrium?

+
In the PhET simulation, you’ll notice that equilibrium is reached when the rate of the forward reaction equals the rate of the reverse reaction, and there is no net change in the concentration of reactants or products.
What does it mean when I adjust a variable, but there’s no change in the equilibrium?

+
If you adjust a variable without observing a change, it might mean the system has adapted to maintain the same equilibrium position. This is known as Le Chatelier’s principle, where the system counteracts the change to re-establish equilibrium.
Can I apply these simulation learnings to real-world scenarios?
+
Yes, understanding reversible reactions through simulation can be directly applied to many practical scenarios, including chemical engineering, environmental science, and biological systems where equilibrium plays a crucial role.